Catalog No.
KAA30301
Description
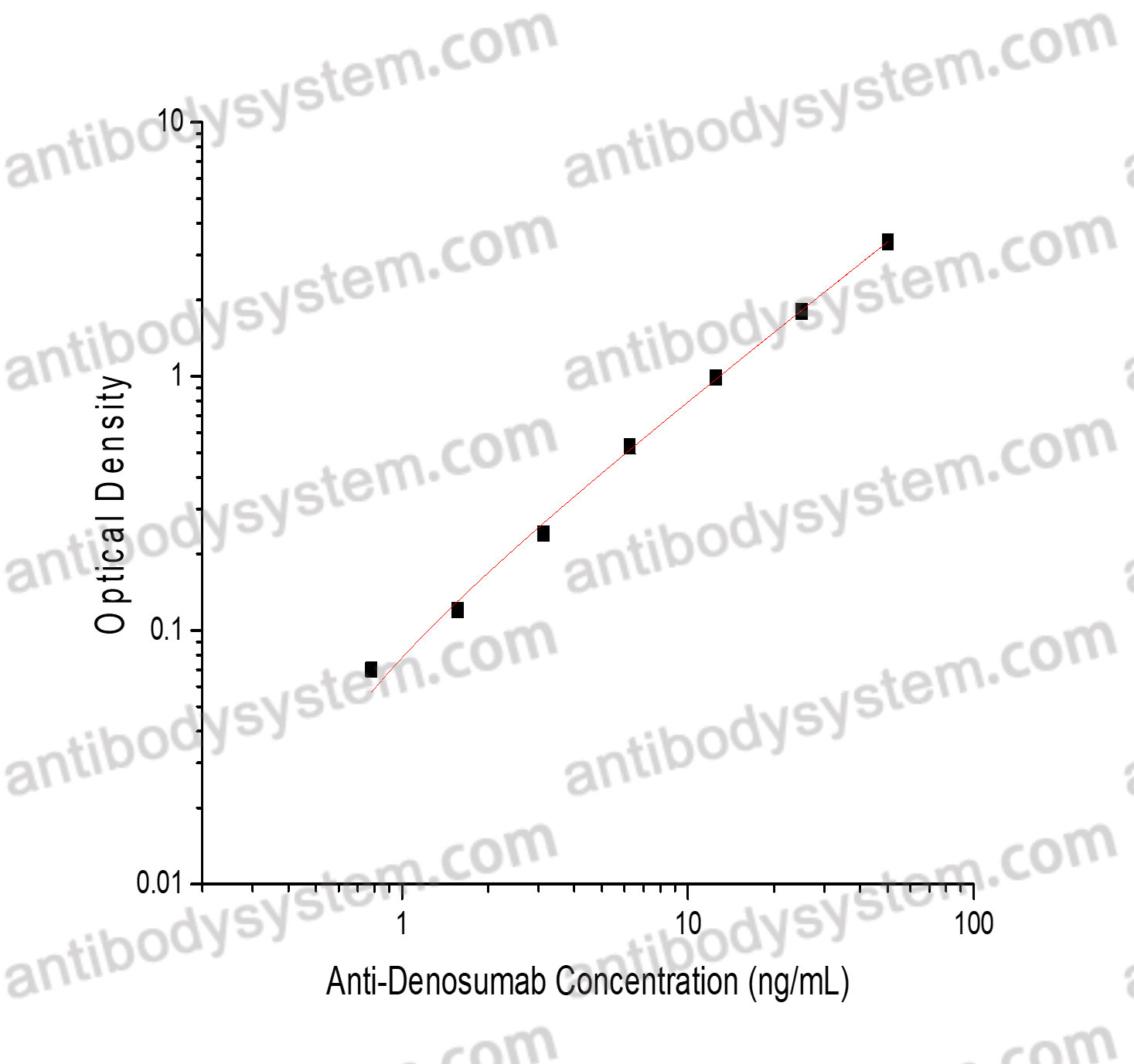
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Denosumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Denosumab will be captured by immobilized Denosumab. After washing away any unbound substances, a biotin-labeled Denosumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Denosumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Denosumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
0.78 - 50 ng/mL
Sensitivity
0.64 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
23.2
|
5.0
|
1.2
|
21.2
|
5.2
|
1.3
|
|
Standard deviation
|
1.3
|
0.3
|
0.1
|
2.7
|
0.5
|
0.1
|
|
CV (%)
|
5.5
|
6.7
|
5.9
|
12.7
|
10.4
|
10.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
AMG162, HL2001,CAS: 615258-40-7
Osteoporosis Management after the Occurrence of Medication-Related Osteonecrosis of the Jaw: A 13-Year Experience at a Tertiary Center., PMID:40509705
Denosumab-bbdz: A Review of the Interchangeable Biosimilar for the Treatment of Osteoporosis., PMID:40509651
RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments., PMID:40507804
Bisphosphonate beak without the bisphosphonate: atypical femoral fracture with denosumab., PMID:40506101
Dental implant failure and medication-related osteonecrosis of the jaw (MRONJ) related to dental implants in patients taking antiresorptive therapy for osteoporosis: a systematic review and meta-analysis., PMID:40505730
Patterns of adjuvant bone modifying agent use in patients with early-stage breast cancer in the United States., PMID:40500761
Prolonged hypercalcemia induced by teriparatide: a case report and literature review., PMID:40500389
Delayed-onset hypocalcaemia and hypophosphataemia induced by iron infusion in the context of denosumab therapy., PMID:40499946
Severe immunotherapy-related thrombocytopenia in metastatic bone cancer: a multicenter retrospective case series highlighting early recognition and management., PMID:40496609
Denosumab in patients with osteogenesis imperfecta and a historical control study with alendronate., PMID:40496553
The prevention of osteoporotic vertebral fractures in eastern and in western countries., PMID:40495909
Fragility of Evidence for the Efficacy of Anti-fracture Medications., PMID:40488289
Moderate-to-severe hypercalcemia secondary to primary hyperparathyroidism refractory to conventional treatment (therapeutic management with denosumab): case report and literature review., PMID:40486005
30 YEARS OF BONE GIANT CELL TUMOR IN THE KNEE: A BRAZILIAN PERSPECTIVE., PMID:40475359
Comparative efficacy of teriparatide and bisphosphonates or denosumab vs. teriparatide monotherapy in osteoporosis: a meta-analysis., PMID:40469971
Diagnosis of skeletal fragility due to Loeys-Dietz syndrome and treatment with romosozumab followed by denosumab., PMID:40469084
Primary Hyperparathyroidism with Severe Vitamin D Deficiency in Chronic Kidney Disease., PMID:40467506
Asia-Pacific consensus for the management of osteoporosis in men., PMID:40464984
Novel approach to central giant cell granuloma of the Jaw: Low-dose intralesional denosumab treatment in a case series of nine patients., PMID:40461338
Impact of denosumab on muscle health in older adults in long-term care., PMID:40460961
Enzalutamide plus radium-223 in metastatic castration-resistant prostate cancer: results of the EORTC 1333/PEACE-3 trial., PMID:40450503
Atypical femoral fracture associated with sequential zoledronic acid and denosumab therapy: a case report., PMID:40459674
A missense mutation in close proximity of ALS-linked PFN1 mutations causes only early-onset Paget's disease of bone., PMID:40458045
Treatment of Osteoporosis in Patients with Chronic Kidney Disease., PMID:40457078
The link between osteoporosis and cardiovascular diseases: a review of shared mechanisms, risk factors, and therapeutic approaches., PMID:40455217
Long-term effectiveness and safety of denosumab for osteoporosis in patients with rheumatic diseases., PMID:40451270
Use of Low-Value Cancer Treatments in Medicare Advantage Versus Traditional Medicare., PMID:40448575
Characteristics and treatment options of 272,152 geriatric patients with very high and high fracture risk., PMID:40445411
Comparison of Type 2 Diabetes Risk in Osteoporosis Patients Treated with Denosumab or Alendronate: A Nationwide Cohort Study., PMID:40445395
EXPRESS: Osteoblasts inhibit NK cell killing function via RANK/RANKL in multiple myeloma., PMID:40444878
A practical use of bone turnover markers in management of patients with skeletal fragility., PMID:40439877
Understanding and Managing Fracture Risk in Patients With Cancer: A Literature Review., PMID:40438830
Five-Year Sales Trends of Osteoporosis Medications in Korea: A Market Analysis Based on IMS Health Sales Audit Data (2018-2023)., PMID:40428763
Supra-Physiological Levels of Magnesium Counteract the Inhibitory Effect of Zoledronate on RANKL-Dependent Osteoclastogenesis., PMID:40427722
Comprehensive Evaluation of Treatment Patterns in Postmenopausal Patients with Osteoporosis without Fractures: Insights from Tertiary Care Institutions and Nationwide OMOP-CDM Data., PMID:40426310
Mouse model of anti-RANKL discontinuation reveals reduced bone mass and quality through disruption of bone remodeling., PMID:40425548
Comparative analysis of Denosumab and Zoledronic acid in advanced breast cancer patients receiving CDK4/6 inhibitors., PMID:40424680
Denosumab and Fracture Prevention in Primary Care Practice., PMID:40423959
Symptomatic hypocalcaemia after administration of denosumab and iron infusion in patients with normal and impaired renal function., PMID:40423532
Role of denosumab in lipid metabolism disorders: clinical significance and potential mechanisms., PMID:40418391
No overshoot of bone turnover after withdrawal of denosumab treatment of adults with Langerhans cell histiocytosis: a prospective clinical trial., PMID:40418338
Evaluation of Anti-SARS-CoV-2 IgG Responses in a Clinical Study of a Biosimilar Candidate to Denosumab Using Singlicate Analysis., PMID:40408051
Effect of denosumab on semen quality in infertile men selected by serum level of antimüllerian hormone: a randomized controlled trial., PMID:40403914
Denosumab treatment of giant cell tumors in the spine induces woven bone formation., PMID:40390810
Real-world differences in denosumab persistence, reinitiation, and switching among cohorts of older adults in Canada and the United States., PMID:40390804
Incidence of medication-related osteonecrosis of the jaw and associated antiresorptive drugs in adult Finnish population., PMID:40389539
A comparative analysis of Denosumab and Zoledronic acid effects on bone metabolism and bone mineral density in individuals with osteoporotic vertebral compression fractures., PMID:40386518
Spinal giant cell tumour presenting as a posterior mediastinal mass., PMID:40379300
Agents to treat osteoporosis in chronic kidney disease., PMID:40377654
Monitoring denosumab therapy using the calcium isotope marker (CIM) technology., PMID:40374024