Catalog No.
KAD17201
Description
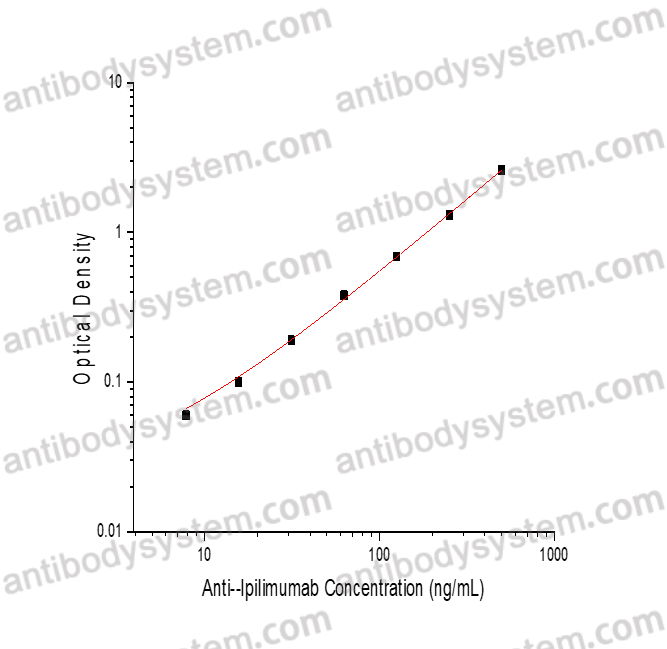
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Ipilimumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Ipilimumab will be captured by immobilized Ipilimumab. After washing away any unbound substances, a biotin-labeled Ipilimumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Ipilimumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Ipilimumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
7.81 - 500 ng/mL
Sensitivity
4.25 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
223.4
|
60.7
|
11.8
|
225.5
|
49.1
|
12.8
|
|
Standard deviation
|
14.0
|
3.3
|
1.1
|
14.9
|
2.8
|
1.0
|
|
CV (%)
|
6.3
|
5.5
|
9.0
|
6.6
|
5.7
|
8.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
BMS-734016,MDX-010,MDX-CTLA-4,CAS: 477202-00-9
Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis., PMID:40507352
Immune Checkpoint Inhibitors in the Treatment of Advanced Melanoma in Older Patients: An Overview of Published Data., PMID:40507314
Gender and age disparities in cardiac immune-related adverse events associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FAERS database., PMID:40506075
Combined immunotherapy with nivolumab and ipilimumab with and without sequential or concomitant stereotactic radiotherapy in patients with melanoma brain metastasis: An international retrospective study., PMID:40505525
NEXUS: a phase I dose escalation study of selinexor plus nivolumab and ipilimumab in Asian patients with advanced/metastatic solid malignancies., PMID:40502306
Biomarker analysis and treatment dynamics following preoperative ipilimumab plus nivolumab in locally advanced urothelial cancer from the phase 1B NABUCCO study., PMID:40489082
Organ-Specific Responses to Nivolumab Plus Ipilimumab in Advanced Hepatocellular Carcinoma: A Multicenter, Retrospective Study., PMID:40488227
CTLA-4 blockade in ovarian cancer immunotherapy: Mechanisms and clinical strategies., PMID:40482550
Cost-Utility and Budget Impact Analysis of Immunotherapy for First-Line Treatment of Advanced Kidney Cancer in Latin America: Evidence From Uruguay., PMID:40482312
A phase II study of nivolumab, ipilimumab, and radiation in combination with influenza vaccine in patients with pancreatic cancer (INFLUENCE)., PMID:40475164
Surgical considerations for tumour-infiltrating lymphocyte therapy in melanoma: results from a randomized phase III trial., PMID:40474841
Treatment-Free Survival Over 6 Years of Follow-up in Patients With Metastatic Non-small Cell Lung Cancer Treated With First-Line Nivolumab Plus Ipilimumab Versus Chemotherapy in CheckMate 227 Part 1., PMID:40473108
Immune-mediated enterocolitis is associated with immune checkpoint inhibitors: A pharmacovigilance study from the FDA Adverse Event Reporting System (FAERS) database., PMID:40465755
Malignant mesothelioma with a novel BAP1 germline frameshift mutation treated with dual immune checkpoint inhibitors: A case report., PMID:40463358
Primary Vaginal Melanoma or Clear Cell Sarcoma: A Difficult Differential Diagnosis., PMID:40462828
Fc-optimized anti-CTLA-4 antibodies increase tumor-associated high endothelial venules and sensitize refractory tumors to PD-1 blockade., PMID:40460830
Combination of encorafenib and binimetinib followed by ipilimumab and nivolumab versus ipilimumab and nivolumab in patients with advanced melanoma with BRAFV600E or BRAFV600K mutations (EBIN): an international, open-label, randomised, controlled, phase 2 study., PMID:40449497
Clinical Management and Outcomes of Immune-Related Adverse Events During Treatment with Immune Checkpoint Inhibitor Therapies in Melanoma and Renal Cell Carcinoma: A UK Real-World Evidence Study., PMID:40448748
Nivolumab plus ipilimumab with chemotherapy as first-line treatment of patients with metastatic non-small-cell lung cancer: final, 6-year outcomes from CheckMate 9LA., PMID:40446626
Long-term real-world outcomes of nivolumab plus ipilimumab for advanced renal cell carcinoma: a minimum of 4-years follow-up study., PMID:40446096
The promise of PD1/PDL1 targeted immunotherapy in locally advanced cervical cancer: a game-changer for patients outcome?, PMID:40433369
Correlation Between Body Mass Index and Clinical Outcomes in Advanced Renal Cell Carcinoma Patients., PMID:40425321
Using liquid biopsies to guide treatment and monitor response in BRAF V600E positive adenocarcinoma of unknown primary., PMID:40425210
Spontaneous Dramatic Regression of Clear Cell Renal Cell Carcinoma After Pazopanib-Induced Severe Systemic Inflammatory Syndrome: A Case Report and Literature Review., PMID:40422519
Multisystem immune-related adverse reactions with immune checkpoint inhibitors., PMID:40421943
Looking Toward the Future: Emerging Therapies for Hepatocellular Carcinoma., PMID:40416920
Case Report: Acral vasculitis induced by Immune Checkpoint Inhibitors: a case series and literature review., PMID:40416866
The Importance of Timing in Immunotherapy: A Systematic Review., PMID:40416194
Adjuvant Immunotherapy in Microsatellite Instability-High Colon Cancer: A Literature Review on Efficacy, Challenges, and Future Directions., PMID:40415356
Systemic Therapy for Melanoma: What Surgeons Need to Know., PMID:40413004
A treatment-related death predictive score for treatment-naïve advanced non-small cell lung cancer., PMID:40409025
A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: durable responses and delayed pseudoprogression in small cell carcinoma of the ovary, hypercalcemic type cohort., PMID:40402690
Integrative Immune Signature of Complementary Circulating and Tumoral Biomarkers Maximizes the Predictive Power of Adjuvant Immunotherapeutic Benefits in High-Risk Melanoma., PMID:40392969
Real-world efficacy and safety of nivolumab and ipilimumab in metastatic renal cell carcinoma: a Polish multicenter study., PMID:40391586
Rationale and feasibility of a rapid integral biomarker program that informs immune-oncology clinical trials: the ADVISE trial., PMID:40389374
Interplay between sarcopenia, GDF-15, and the efficacy of nivolumab plus ipilimumab in patients with mismatch repair deficient metastatic colorectal cancer: final survival analysis of the phase II GERCOR NIPICOL study., PMID:40389373
Metabolic tumor volume on 18F-FDG uptake as a negative predictor after ipilimumab plus nivolumab in advanced non-small cell lung cancer., PMID:40386730
Breaking Through: Immunotherapy Innovations in Pleural Mesothelioma., PMID:40382268
Phase II Study (NO LIMIT, WJOG13320G) of First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High Advanced Gastric or Esophagogastric Junction Cancer., PMID:40378356
[Renal injury during combination immunotherapy (ipilimumab + nivolumab) in patients with metastatic renal cell cancer who previously underwent nephrectomy]., PMID:40377638
Exceptional Response in a Patient with mRCC Through Precision-Guided Treatment Involving Immunotherapy Rechallenge with Temsirolimus and Bevacizumab., PMID:40376550
Association of Sinonasal Symptoms and Disease With Immune Checkpoint Inhibitor Therapy., PMID:40370338
Immune Checkpoint Inhibitor-Associated Acute Kidney Injury: A Single-Center Experience of Biopsy-Proven Cases., PMID:40364262
Haematological toxicities with immune checkpoint inhibitors in digestive system tumors: a systematic review and network meta-analysis of randomized controlled trials., PMID:40360867
Nivolumab plus low-dose ipilimumab in hypermutated HER2-negative metastatic breast cancer: a phase II trial (NIMBUS)., PMID:40360544
An Extremely Rare Case of Immune Checkpoint Inhibitors-Related Hypoparathyroidism and a Critical Literature Review., PMID:40356787
Accelerated atherosclerosis associated with immune checkpoint inhibitors: a systematic review and meta-analysis of pre-clinical studies., PMID:40354680
Adverse neurologic events of immune checkpoint inhibitor monotherapy vs. combination therapy for melanoma., PMID:40351833
Nivolumab plus ipilimumab in hepatocellular carcinoma., PMID:40349715
Nivolumab plus ipilimumab versus lenvatinib or sorafenib as first-line treatment for unresectable hepatocellular carcinoma (CheckMate 9DW): an open-label, randomised, phase 3 trial., PMID:40349714