Catalog No.
KAC90701
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Rituximab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Rituximab will be captured by immobilized Rituximab. After washing away any unbound substances, a biotin-labeled Rituximab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Rituximab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Rituximab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
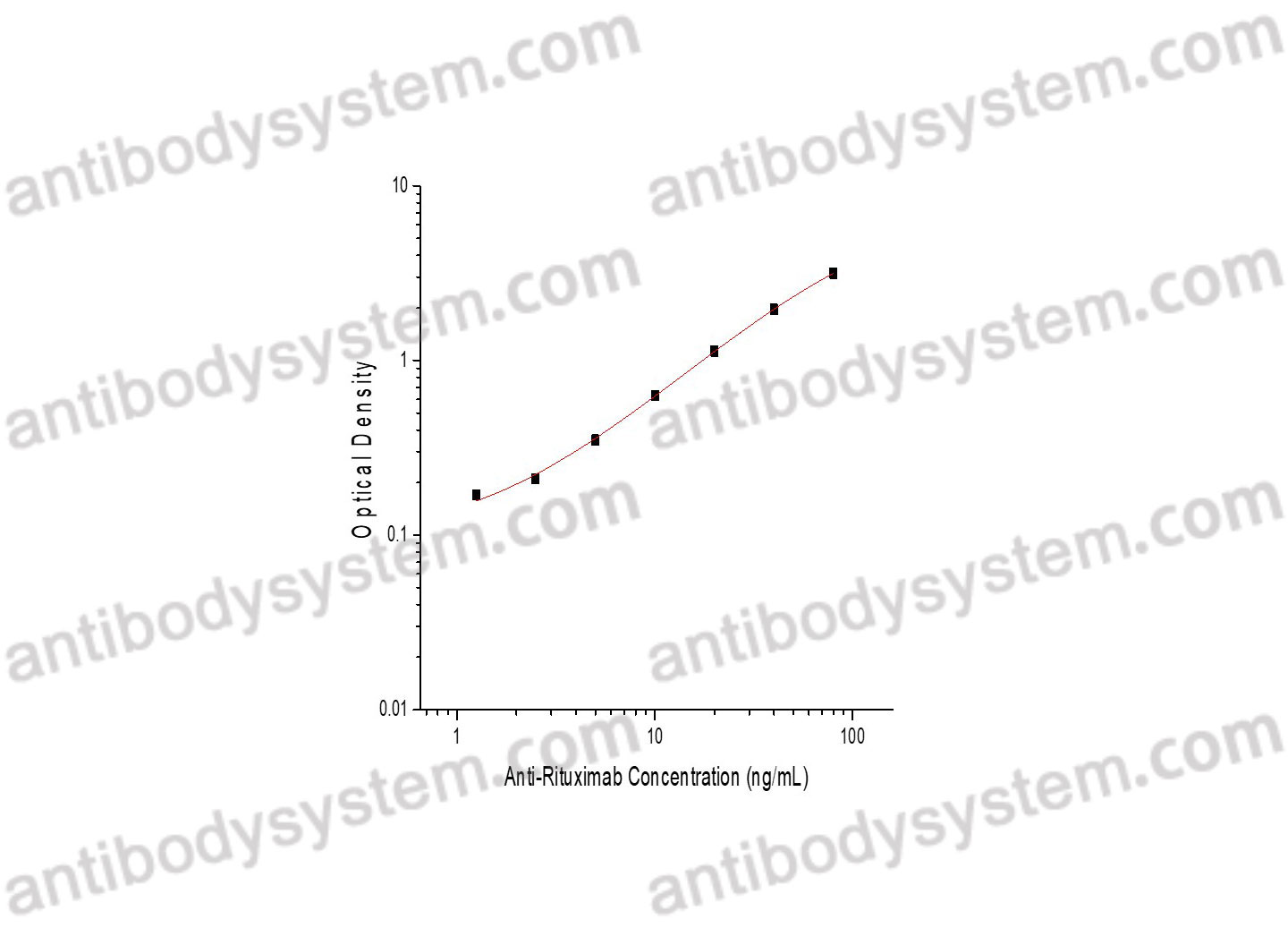
Range
1.25 - 80 ng/mL
Sensitivity
0.89 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
32.1
|
8.6
|
2.1
|
32.2
|
8.8
|
2.4
|
|
Standard deviation
|
0.8
|
0.2
|
0.1
|
1.7
|
0.3
|
0.2
|
|
CV (%)
|
2.5
|
2.3
|
4.2
|
5.1
|
3.0
|
8.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
IDEC-C2B8,CAS: 174722-31-7
Response to telitacicept in optic neuritis associated with Sjogren's syndrome: a case report and literature review., PMID:40515854
Pharmacokinetic-pharmacodynamic modelling in a clinical pilot study of rituximab in multiple sclerosis: Towards personalized dosing interval., PMID:40515509
Medicare spending and use of subcutaneous biologic formulations with hyaluronidase., PMID:40515473
Clinical and Electrodiagnostic Findings in Anti-myelin-Associated Glycoprotein Antibody Polyneuropathy: A Single Center Review., PMID:40513035
Long-Term Outcomes of Rituximab Therapy in Autoimmune Hemolytic Anemia: A Systematic Review and Meta-Analysis., PMID:40510077
Therapeutic Challenges and New Era in Fibrillary Glomerulonephritis with the Introduction of DNAJB9: Experience from a Tertiary Nephrology Center., PMID:40507471
A Triple Oral Combination of Bendamustine, Acalabrutinib, and Venetoclax Demonstrates Efficacy Against Mantle Cell Lymphoma In Vitro and In Vivo., PMID:40507368
Extravascular leakage of dexrazoxane that occurred in a patient with diffuse large B-cell lymphoma: a case report., PMID:40506789
Efficacy of rituximab versus cyclophosphamide in connective tissue disease‑related interstitial lung disease: a systematic review and meta-analysis., PMID:40506621
Emerging Role of Targeted Monoclonal Antibodies in Neuromyelitis Optica Spectrum Disorders., PMID:40506618
Abatacept and multiple therapeutic bronchoscopies as salvage therapy for refractory tracheobronchial inflammation and stenosis in GPA., PMID:40506105
Effectiveness of anti-CD20 therapies following natalizumab discontinuation: insights from a cohort study., PMID:40505538
Relapse and beyond: Navigating the long-term clinical impacts of immune thrombotic thrombocytopenic purpura., PMID:40505364
FcRn inhibitors in immune thrombocytopenia: A comprehensive review of therapeutic advances and clinical outcomes., PMID:40505332
A single dose of a CD137 antibody-drug conjugate protects nonhuman primate allogeneic HCT recipients against acute GVHD., PMID:40504994
Synchronous MALT lymphoma and gastric adenocarcinoma., PMID:40503979
Diagnosis and treatment of metachronous multiple primary carcinoma: A case report and review of literature., PMID:40503404
Rituximab-based regimens for primary cardiac lymphoma: A systematic review of outcomes, challenges and future directions., PMID:40503399
Investigating outcomes and treatment of long coronavirus disease 2019 in patients with multiple sclerosis treated with rituximab: A case series study., PMID:40503046
Current Status of Pig-to-NHP Xenotransplantation Research in Korea., PMID:40500644
Clinical uptake of an antigen-based approach to membranous nephropathy: a survey of general nephrologists and glomerular disease experts., PMID:40500560
Combination therapy of rituximab and mycophenolate in patients with systemic sclerosis and primary cardiac involvement refractory to cyclophosphamide: a retrospective exploratory analysis of 10 cases., PMID:40499976
Mathematical modeling of Taylor-Aris dispersion-assisted mass spectrometry for the study of operating conditions., PMID:40499387
Copanlisib in combination with rituximab and bendamustine for transplant-ineligible relapsed/refractory diffuse large B-cell lymphoma patients: Results from the phase II multicentre FIL Copa-BR trial from Fondazione Italiana Linfomi (FIL)., PMID:40495420
Mycosis Fungoides, Sézary Syndrome, and Cutaneous B-Cell Lymphomas: 2025 Update on Diagnosis, Risk-Stratification, and Management., PMID:40495407
Comparison and analysis of biological drug consumption in two Italian hospital settings: Governance actions and prescribing appropriateness., PMID:40494065
Prognosis of essential mixed cryoglobulinemia and connective tissue disease-related cryoglobulinemia after rituximab-induced remission., PMID:40493895
A 14-year girl with thrombotic thrombocytopenic purpura and Sjögren's syndrome., PMID:40492669
Factors for Rituximab Refractoriness in AQP4-IgG+ NMOSD: A Cohort Study., PMID:40492601
Economic Evaluation of Rituximab Versus Corticosteroid-Cyclophosphamide or Cyclosporine in Patients With Membranous Nephropathy in Republic of Korea., PMID:40492539
Rituximab as First-Line Compared to Escalation Immunotherapy Is Associated With Lower Disability Accumulation in Aquaporin-4-IgG-Positive Neuromyelitis Optica Spectrum Disorder: A Multicenter Cohort Study From Germany and the United Kingdom., PMID:40492480
CD20 targeted nanomedicine for GCB-diffuse large B-cell lymphoma through synergistic effects of apoptosis and ferroptosis., PMID:40492153
Dual BLyS/APRIL targeted therapy with telitacicept in rituximab-refractory SLE-associated neuromyelitis optica spectrum disorder: a case report., PMID:40491913
Therapeutic Choices in Systemic Sclerosis-Associated Interstitial Lung Disease, a Survey of 2 International Research Groups., PMID:40489954
Monitoring anti-Rituximab antibodies in myasthenia gravis affects the time to event during Rituximab treatment., PMID:40488894
Safety of glucocorticoid dose reduction in microscopic polyangiitis: a multicentre REVEAL cohort study., PMID:40488413
Nation-wide cohort study of Japanese patients with ANCA-associated vasculitis using rituximab: effectiveness and safety after two years., PMID:40488401
Extranodal Marginal Zone Lymphoma Presenting as Acute Kidney Injury due to Cast Nephropathy: A Case Report., PMID:40487070
Advances and challenges in leukemia treatment: A focus on monoclonal antibodies and emerging therapies., PMID:40486885
Dual Autoimmunity: A Case Report of the Sequential Development of Systemic Lupus Erythematosus in a Patient With Anti-MDA5 Dermatomyositis., PMID:40486387
Treatment of Nephrotic Syndrome With Antinephrin Antibodies Using Plasmapheresis, Rituximab, and Mycophenolate Mofetil., PMID:40485695
Persistent B Cell Depletion After Rituximab for Autoimmune and Glomerular Diseases: A Case Series., PMID:40485690
What Is the Evidence on Immunomodulators and Immunosuppressants for Progressive Multiple Sclerosis? - A Cochrane Review Summary with Commentary., PMID:40485316
How well does acalabrutinib work and how safe is it to treat patients with chronic lymphocytic leukemia/small lymphocytic lymphoma who have had previous treatments? a plain language summary of 2 key studies., PMID:40485230
[Diagnosis, treatment, and genetic analysis of five cases of primary atypical hemolytic uremic syndrome]., PMID:40484823
[Clinical characteristics and outcomes of elderly patients with stage Ⅰ diffuse large B-cell lymphoma: a study by the Jiangsu Cooperative Lymphoma Group (JCLG)]., PMID:40484818
Complex presentation of probable catastrophic antiphospholipid syndrome: diagnostic dilemmas and treatment strategies., PMID:40484440
Role of Chimeric Antigen Receptor-Expressing Cell Therapy in Immune-Mediated Kidney Diseases: A Review., PMID:40484342
Long-term maintenance of remission with spacing of rituximab infusions based on the individualised patient risk profile in ANCA-associated vasculitis: a pilot study., PMID:40480651
Haemophagocytic lymphohistiocytosis (HLH) secondary to measles in an adult with a loss of post-vaccination humoral immunity following rituximab., PMID:40480240