Catalog No.
KAD62601
Description
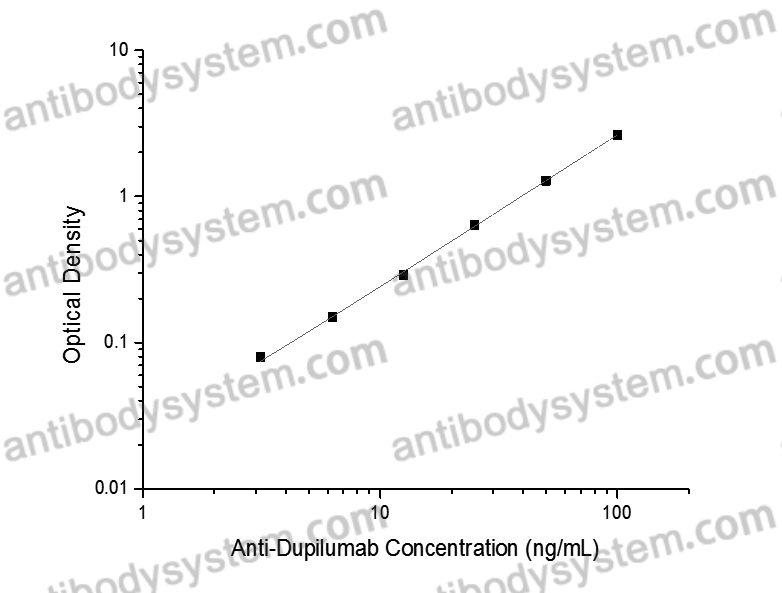
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Dupilumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and anti-Dupilumab will be captured by immobilized Dupilumab. After washing away any unbound substances, a biotin-labeled Dupilumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Dupilumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Dupilumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
3.13 - 100 ng/mL
Sensitivity
1.32 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
59.4
|
28.7
|
5.2
|
63.5
|
28.9
|
7.4
|
|
Standard deviation
|
5.5
|
1.9
|
0.5
|
9.4
|
2.7
|
1.0
|
|
CV (%)
|
9.3
|
6.7
|
8.9
|
14.7
|
9.4
|
12.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
CD124,REGN668,SAR231893, CAS: 1190264-60-8
The Efficacy of Dupilumab in Pemphigus Vulgaris., PMID:40514796
Clinical response and remission in patients treated with dupilumab for severe asthma: Results from the nationwide Danish Severe Asthma Register., PMID:40513967
Recalcitrant Grover's disease treated with dupilumab: two long-term cases and review of the literature., PMID:40509692
Efficacy of Upadacitinib in Treating Alopecia Areata, Atopic Dermatitis, and Th1 Comorbidities in Pediatric Patients: A Comprehensive Case Series and Literature Review., PMID:40507644
Dupilumab-Induced Remission in Chronic Rhinosinusitis with Nasal Polyps and Comorbid Asthma: A 24-Month Study., PMID:40507416
Interleukin-4 and -13 Gene Expression Profiles in Immune-Related Bullous Pemphigoid Indicate Efficacy of IL-4/IL-13 Inhibitors., PMID:40507326
Dupilumab in an adolescent with eosinophilic esophagitis and eosinophilic duodenitis, a valuable treatment option., PMID:40504588
Dupilumab treatment has no effect on the nasal microbiome in patients with NSAID-exacerbated respiratory disease: a longitudinal pilot study., PMID:40503236
STAT6 gain-of-function disease: p.D519N is a new disease-causing variant that responds well to dupilumab treatment., PMID:40502541
Paradoxical Effects of Dupilumab in ABPA: Resolution of Mucus Plugging but Induction of Eosinophilic Pneumonia., PMID:40499647
Auditory and Ocular Manifestations in Pediatric Vitiligo., PMID:40498511
Dupilumab and Blood Eosinophilia: A Disease-Specific Phenomenon?, PMID:40492692
[Application and research progress of anti-IL-4α monoclonal antibody dupilumab in the treatment of type 2 asthma]., PMID:40491151
Eosinophilic esophagitis in children: new kids on the block., PMID:40490426
Aspirin-Exacerbated Respiratory Disease in the era of biologics., PMID:40490219
Drug-related visual blurring: findings from the U.S. Food and Drug Administration Adverse Event Reporting System database., PMID:40490170
Wells syndrome: emerging triggers and treatments- an updated systematic review., PMID:40488888
Effectiveness of Dupilumab Treatment in Patients With Type 2 Inflammation: A Real-Life Integrated Experience., PMID:40487737
Dupilumab-Induced Granuloma Annulare: A Challenging Adverse Effect of Atopic Dermatitis Treatment., PMID:40487493
Dupilumab Improves Histopathologic Features in Patients With Eosinophilic Esophagitis: LIBERTY EoE TREET Study Results., PMID:40487268
Advancements in Biologic Therapies for Pediatric Asthma: Emerging Therapies and Future Directions., PMID:40486460
Cost-utility analysis of sequential therapies for pediatric-onset eosinophilic esophagitis., PMID:40485534
A nationwide experience of biological treatments in children with eosinophilic esophagitis., PMID:40483369
Reply to "Considerations for enhancing dupilumab research in hypereosinophilic syndromes"., PMID:40483017
Considerations for enhancing dupilumab research in hypereosinophilic syndromes., PMID:40483016
Clinical and complete remission in patients with severe asthma with 24-month dupilumab treatment., PMID:40482373
Long-term effectiveness and safety of dupilumab in severe asthma: a 5-year real-world multicenter study., PMID:40480546
Real-World Efficacy and Safety of Dupilumab Retreatment in Adults With Relapsed Atopic Dermatitis: A Retrospective Study From China., PMID:40474541
Efficacy and glucocorticoid sparing of dupilumab in IgG4-related disease: a case series and literature review., PMID:40470558
Angiolymphoid hyperplasia with eosinophilia treated with dupilumab in a biologic-naïve patient., PMID:40466455
Effect of dupilumab for the therapy of atopic dermatitis and the effect on serum TARC, HBD2 and SEA-IgE levels and peripheral blood T helper cell subtypes., PMID:40462855
Real‑World Experience of 1 Year of Tralokinumab Treatment in Adult Patients with Atopic Dermatitis: A Single-Center Retrospective Study., PMID:40459712
Real-World Effectiveness and Safety of Dupilumab, Tralokinumab, and Upadacitinib in Patients with Atopic Dermatitis: A 52-Week International, Multicenter Retrospective Cohort Study., PMID:40459711
Dupilumab efficacy in patients with type 2 asthma and early Feno level reductions., PMID:40458676
Rapid Itch Improvement and Skin Clearance with Upadacitinib Versus Placebo (Measure Up 1 and Measure Up 2) and Versus Dupilumab (Heads Up): Results from Three Phase 3 Clinical Trials in Patients with Moderate-to-Severe Atopic Dermatitis., PMID:40457140
An unbiased tissue transcriptome analysis identifies potential markers for skin phenotypes and therapeutic responses in atopic dermatitis., PMID:40456762
[Not Available]., PMID:40456219
[Not Available]., PMID:40456218
Dupilumab Treatment Is Associated With Clinical Improvement and a Shift Toward a Health-Associated Nasal Passage Microbiota in Diffuse Type 2 Chronic Rhinosinusitis., PMID:40452334
Infectious complications of atopic dermatitis, an update., PMID:40447099
[Translated article] Exploring New Horizons in Allergic Contact Dermatitis Treatment: The Role of Emerging Therapies., PMID:40446917
Impacto de dupilumab en la calidad de vida, ansiedad y depresión en pacientes adultos afectados por dermatitis atópica moderada-severa y sus convivientes: un estudio prospectivo., PMID:40446914
[[Translated article]]Treatment of Bullous Pemphigoid with Dupilumab: Retrospective, Single-Center Study in a Spanish Tertiary Referral Center., PMID:40446909
Real-World Clinical Experience of Tralokinumab in a Tertiary Centre: An Alternative for Patients with Conjunctivitis on Dupilumab?, PMID:40442566
Efficacy and safety of dupilumab in pediatric patients aged 6 months to 11 years with erythrodermic atopic dermatitis: A post-hoc analysis of three clinical trials., PMID:40441387
Egg Oral Immunotherapy With Dupilumab Premedication in an Adult Patient., PMID:40440105
Contemporary Concise Review 2024: Chronic Obstructive Pulmonary Disease., PMID:40437348
Type 2 cytokines pleiotropically modulate sensory nerve architecture and neuroimmune interactions to mediate itch., PMID:40436117
Joint Pain and Inflammatory Arthropathy after Dupilumab Use in Atopic Dermatitis: A Systematic Review and Meta-Analysis., PMID:40435582
Real world evidence of dupilumab effectiveness in a Colombian cohort of patients diagnosed with severe asthma., PMID:40432729