Catalog No.
KAH02202
Description
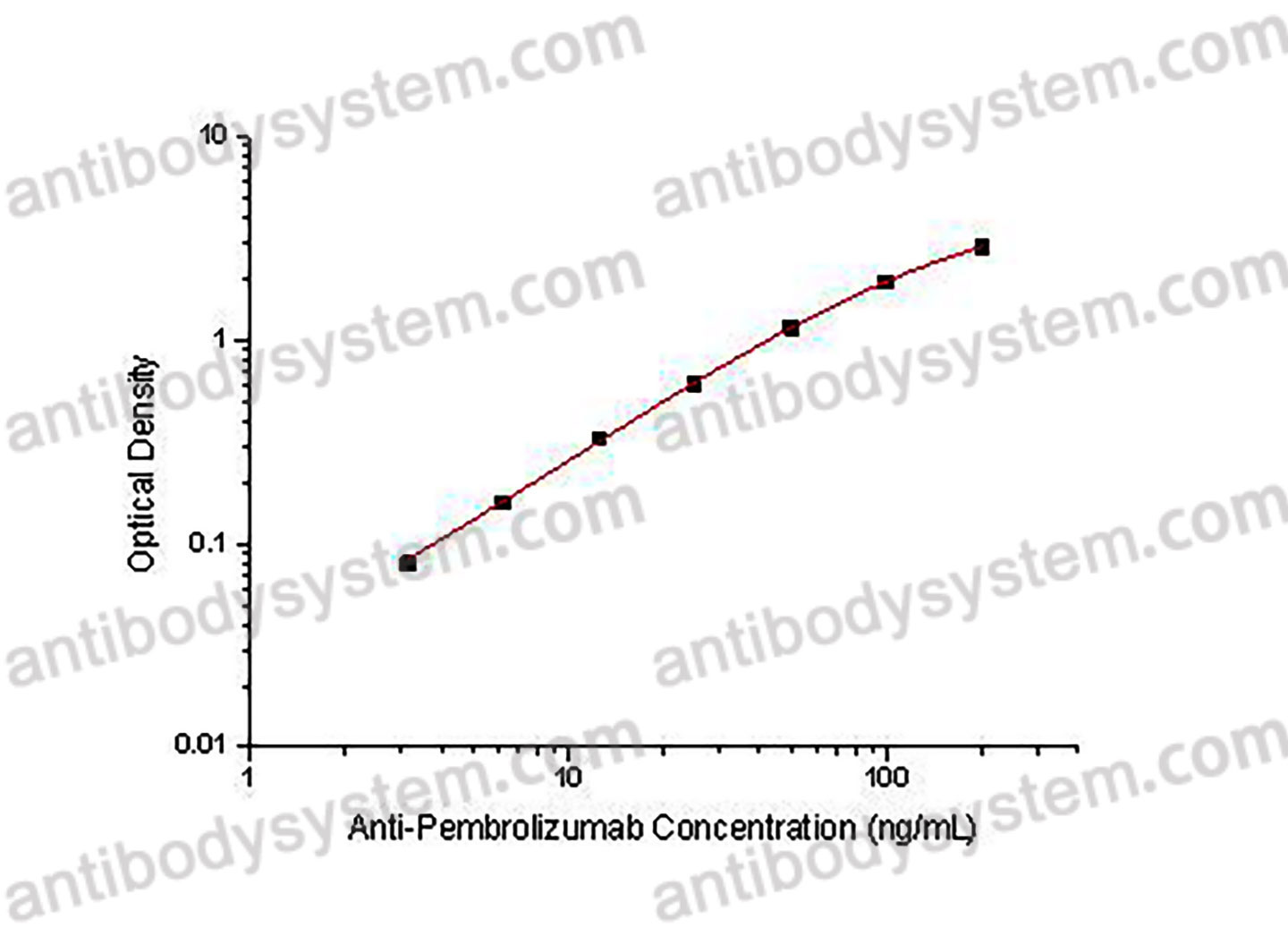
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Pembrolizumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Pembrolizumab will be captured by immobilized Pembrolizumab. After washing away any unbound substances, a biotin-labeled Pembrolizumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Pembrolizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Pembrolizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
3.13 - 200 ng/mL
Sensitivity
1.96 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
94.6
|
24.4
|
5.9
|
97.5
|
23.1
|
5.1
|
|
Standard deviation
|
3.5
|
1.0
|
0.5
|
4.1
|
1.7
|
0.5
|
|
CV (%)
|
3.7
|
4.0
|
9.0
|
4.2
|
7.2
|
9.8
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MK-3475, CAS: 1374853-91-4
Cardiac outcomes following adjuvant radiotherapy in triple-negative breast cancer patients with prior autoimmune myocarditis., PMID:40527411
Efficacy of pembrolizumab in MSI-high and BRCA-positive castration-resistant prostate cancer., PMID:40527113
Wolf isotopic response: immunotherapy-related lichenoid eruption localizing to prior radiation site., PMID:40526967
Enfortumab vedotin-induced widespread vesiculobullous eruption mimicking disseminated herpetic infection in a patient with metastatic urothelial carcinoma., PMID:40526955
Optimizing enfortumab vedotin plus pembrolizumab therapy., PMID:40526099
Predicting outcomes with pembrolizumab: a meta-analysis of pre-treatment hematological and clinical prognostic factors in advanced/metastatic urothelial carcinoma., PMID:40526042
Immune Checkpoint Inhibitors for Microsatellite Instability High Unresectable Obstructive Colon Cancer: A Report of Two Cases., PMID:40524862
Adjuvant pembrolizumab therapy for completely resected stage I lung adenocarcinoma with micropapillary or solid histological subtypes: a single-center, single-arm, phase 2 trial., PMID:40524802
Aortoesophageal fistula following thoracic branch endoprosthesis for cryptogenic penetrating aortic ulcer in a patient on pembrolizumab., PMID:40521387
Synergistic approach to combating triple-negative breast cancer: DDR1-targeted antibody-drug conjugate combined with pembrolizumab., PMID:40521369
A pilot study investigating the effect of pembrolizumab on the tumoral immunoprofile of newly diagnosed mullerian cancers., PMID:40521348
Immune-Related Fulminant Myocarditis Revealed Using Myocardial Histopathology at Autopsy in the Treatment of Advanced Renal Cell Carcinoma: A Case Report., PMID:40519645
The Efficacy and Safety of Pembrolizumab in Anaplastic Thyroid Carcinoma: A Systematic Review and Meta-Analysis., PMID:40518758
FDG-PET/CT and CT compared for evaluation of tumor response to first-line immunotherapy and prediction of prognosis in non-small-cell lung cancer patients., PMID:40517759
Uncovering prognostic biomarkers through a pharmacovigilance study: the case of RDW., PMID:40517345
Current status of adjuvant immunotherapy and relapse management in renal cell carcinoma: Insights from a European delphi study., PMID:40516320
Avelumab maintenance in advanced urothelial carcinoma: real-world data from Northern Spain (AVEBLADDER study)., PMID:40514610
Nivolumab plus relatlimab in advanced melanoma: RELATIVITY-047 4-year update., PMID:40513285
Neoadjuvant dose-dense anthracycline and cyclophosphamide in combination with carboplatin, paclitaxel, and pembrolizumab for triple-negative breast cancer: a systematic review and meta-analysis., PMID:40512324
LiGeR-HN phase III trials of petosemtamab + pembrolizumab and petosemtamab monotherapy in recurrent or metastatic HNSCC., PMID:40511820
Pembrolizumab-induced myocarditis, myositis, and myasthenia gravis overlap syndrome (IM3OS) treated with Efgartigimod: case report., PMID:40510022
Current Progress and Future Perspectives of RNA-Based Cancer Vaccines: A 2025 Update., PMID:40507360
Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis., PMID:40507352
Immune Checkpoint Inhibitors in the Treatment of Advanced Melanoma in Older Patients: An Overview of Published Data., PMID:40507314
Baseline Radiomics as a Prognostic Tool for Clinical Benefit from Immune Checkpoint Inhibition in Inoperable NSCLC Without Activating Mutations., PMID:40507271
Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy., PMID:40507249
Pembrolizumab plus chemotherapy in advanced endometrial cancer: a cost-effectiveness analysis., PMID:40506696
[Lichen sclerosus induced by pembrolizumab]., PMID:40506272
Management of Cutaneous Melanoma, With a Special Focus on Desmoplastic Melanoma., PMID:40505073
Adjuvant pembrolizumab following kidney cancer surgery A novel patient decision aid to facilitate shared decision-making., PMID:40503866
Managing severe dermatitis and grade 4 pneumonitis in a patient with non-small-cell lung cancer following pembrolizumab treatment: A case report., PMID:40503034
Metastatic renal cell carcinoma to the glans penis: A case report of rare presentation., PMID:40502920
Design and rationale of the phase II PANDORA trial: first line chemo-immunotherapy in advanced Merkel cell carcinoma., PMID:40501309
Phase 1 and 2 Clinical Studies of the STING Agonist Ulevostinag With and Without Pembrolizumab in Participants With Advanced or Metastatic Solid Tumors or Lymphomas., PMID:40499147
Impact of Tumor Response and Response Duration on Survival Among Participants Receiving Pembrolizumab Plus Chemotherapy as First-Line Therapy for Non-Small-Cell Lung Cancer., PMID:40498298
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
Enfortumab vedotin induced interstitial lung disease: A first case report with pathological evidence from transbronchial lung cryobiopsy., PMID:40496844
Severe immunotherapy-related thrombocytopenia in metastatic bone cancer: a multicenter retrospective case series highlighting early recognition and management., PMID:40496609
First-line pembrolizumab used concurrently with multi-agent chemotherapy and inhaled tranexamic acid (TXA) for management of stage III mixed trophoblastic tumor complicated by pulmonary hemorrhage., PMID:40496174
Response of EGFR-mutated pulmonary pleomorphic carcinoma to pembrolizumab., PMID:40491684
Pembrolizumab-induced toxic epidermal necrolysis with limited mucosal involvement., PMID:40491518
First-Line Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Retrospective, Multicenter, Real-World Study., PMID:40491285
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
Cost-effectiveness of chemotherapy in advanced and recurrent endometrial cancer., PMID:40487854
A Phase 1/1B Trial of Pembrolizumab and Trametinib in Advanced NSCLC Enriched for KRAS Mutations., PMID:40486486
Second-line pembrolizumab for advanced HCC: Meta-analysis of the phase III KEYNOTE-240 and KEYNOTE-394 studies., PMID:40486134
Cost-Utility and Budget Impact Analysis of Immunotherapy for First-Line Treatment of Advanced Kidney Cancer in Latin America: Evidence From Uruguay., PMID:40482312
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of belantamab mafodotin for multiple myeloma, asciminib for leukemia, osimertinib for lung cancer, amivantamab for lung cancer, and pembrolizumab for pleural mesothelioma in Japan., PMID:40481943
Two Cases of Enfortumab Vedotin-Induced Drug Eruption Diagnosed Based on the Distinctive Epidermal Mitotic Figures in Patients Receiving Combination Therapy With Pembrolizumab., PMID:40476598
Immune checkpoint inhibitor therapy in metastatic renal cell carcinoma: tumour response and immune-related renal vasculitis following cytoreductive nephrectomy., PMID:40474803