Catalog No.
KAB94405
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Etanercept has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Etanercept will be captured by immobilized Etanercept. After washing away any unbound substances, a biotin-labeled Etanercept is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Etanercept bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Etanercept concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
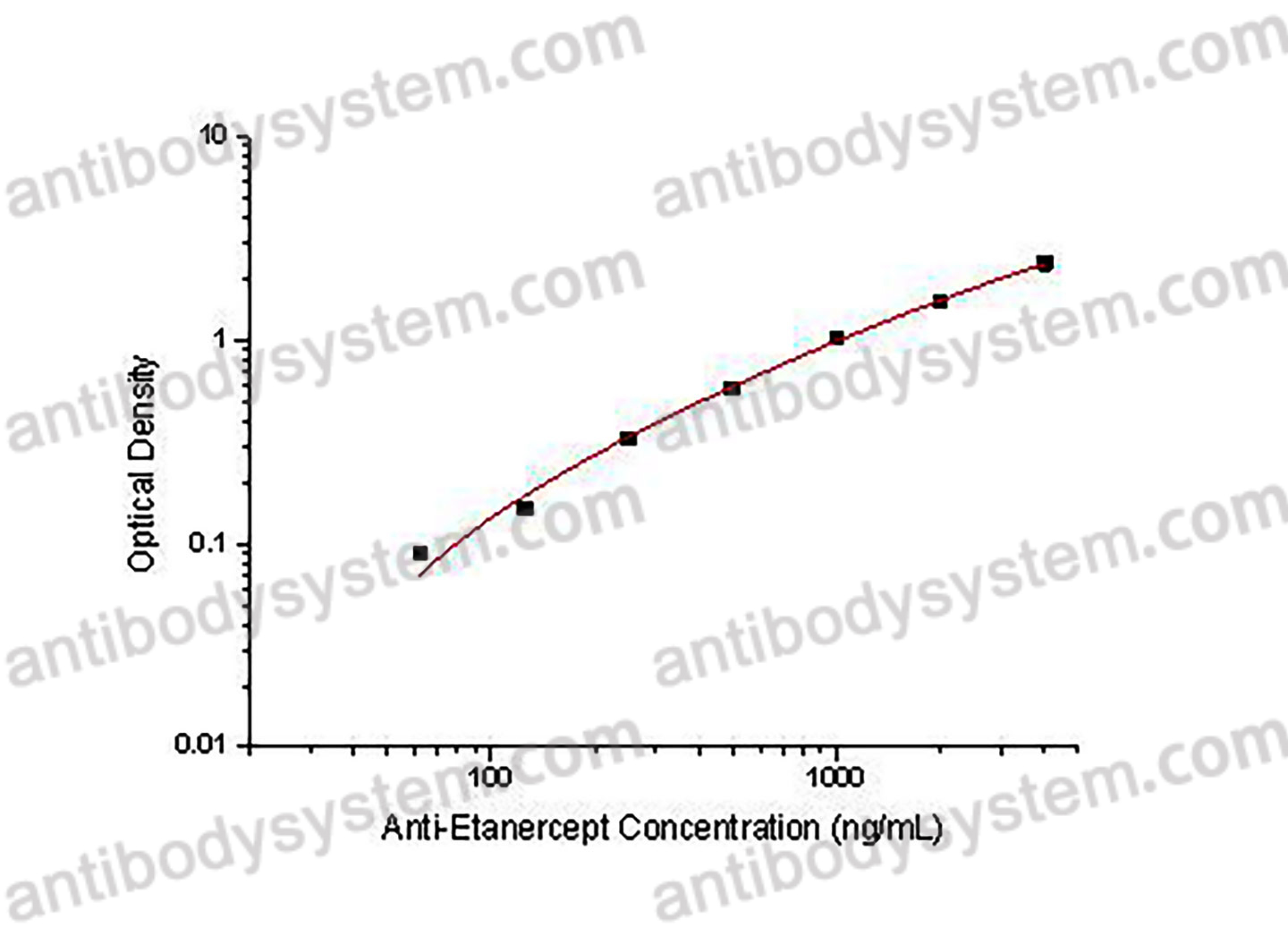
Range
62.5 - 4,000 ng/mL
Sensitivity
27.96 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
926.8
|
221.4
|
103.7
|
919.3
|
230.0
|
95.4
|
|
Standard deviation
|
39.0
|
9.1
|
8.7
|
68.6
|
13.3
|
13.7
|
|
CV (%)
|
4.2
|
4.1
|
8.4
|
7.5
|
5.8
|
14.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CAS: 185243-69-0
Comparison and analysis of biological drug consumption in two Italian hospital settings: Governance actions and prescribing appropriateness., PMID:40494065
Total Knee Arthroplasty in a Patient With Isolated Rheumatoid Arthritis of the Knee., PMID:40491655
Drug survival, long-term safety and efficacy of anti-TNF-alpha biosimilars: a monocentric retrospective study., PMID:40485573
Pharmacogenomics of TNF inhibitors., PMID:40469293
Evaluation of Factors Associated With Uveitis Onset Timing in Oligoarticular Juvenile Idiopathic Arthritis., PMID:40454975
Etanercept, a peripherally restricted TNF inhibitor, enhances cocaine-induced locomotor behaviors in male, but not female, rats., PMID:40446442
Psoriasis complicated with polymyositis successfully treated with Ixekizumab: A case report., PMID:40441209
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Predicting Clinical Response to Monoclonal TNF Inhibitors in Rheumatoid Arthritis: A Transcriptomic Approach Based on Transmembrane TNF Reverse Signaling and Nrf2 Activation., PMID:40428231
Comparative efficacy, safety and immunogenicity of biosimilars and their reference biologic drugs in ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials., PMID:40418343
The Relationship Between TNF-α Inhibitor Potency and HBV Reactivation in Patients With Rheumatic Disorders., PMID:40418092
Rapid baseline hump-free analysis of therapeutic proteins in a wide molecular weight range by SDS - capillary agarose gel electrophoresis., PMID:40409905
Coexistent Ankylosing Spondylitis and Ocular Toxocariasis in a Pediatric Patient Manifesting As Bilateral Panuveitis., PMID:40406765
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Dual therapy for amanita phalloides-induced acute liver failure in mice: A combination of etanercept and alpha-1 antitrypsin., PMID:40398510
Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis., PMID:40393718
Identification of therapeutic targets for neonatal respiratory distress: A systematic druggable genome-wide Mendelian randomization., PMID:40388790
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
Ethanol-induced dysfunction of the mesenteric perivascular adipose tissue is driven by mineralocorticoid receptors., PMID:40377659
Cardiovascular Outcomes of Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis: A Review of the Current Evidence., PMID:40376321
Efficacy of Tumor Necrosis Factor Inhibitors for Refractory Leg Ulcers in Cutaneous Polyarteritis Nodosa: A Case Series., PMID:40374513
Drug survival and predictor factors for discontinuation of first-line biologic therapy in rheumatoid arthritis: data from a real-world single-centre study., PMID:40371548
Drug-associated infections and infestations in older adults with tumor necrosis factor-alpha inhibitors: a real-world retrospective and pharmacovigilance study., PMID:40371340
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Assessing real-world treatment with SDZ ETN (an etanercept biosimilar) in people with rheumatic diseases included in the COMPACT study: a plain language summary., PMID:40353738
Subclinical Enthesitis in Children with Chronic Nonbacterial Osteomyelitis., PMID:40353601
Aggregate Distributional Cost-Effectiveness Analysis of Biologics for the Treatment of Ankylosing Spondylitis in Chile., PMID:40329067
Compatibility between Proteins and Polysaccharide Excipients in Oral Delivery Tablets., PMID:40323221
TNF-α antagonist alleviates muscular layer enlargement but does not prevent myenteric neuronal loss in the colon of streptozotocin-induced diabetic rats., PMID:40288461
Impact of excess weight on clinical features of psoriasis and efficacy of biologic therapies in children with severe psoriasis: Analysis of data from the BiPe cohorts., PMID:40286464
Patient-Reported Outcome Measures in Patients with Rheumatoid Arthritis, Psoriasis, or Psoriatic Arthritis Treated with GP2015, an Etanercept Biosimilar: Results from Two Phase III Studies (EGALITY and EQUIRA)., PMID:40279006
The impact of biosimilar use on healthcare utilization among new users of etanercept for inflammatory arthritis: a population-based regression discontinuity analysis., PMID:40270586
Predicting extra-musculoskeletal and peripheral manifestations and their role on biologic treatment in patients with axial spondyloarthritis: TReasure experience., PMID:40264484
Recommendations for the use of DMARDs in pregnancy and reproductive health for patients with rheumatic disease: A scoping review., PMID:40256995
Divergent disruptive effects of soluble recombinant tau assemblies on synaptic plasticity in vivo., PMID:40251677
Characterising infusion/injection-related reactions in patients with rheumatoid arthritis treated with biologic agents., PMID:40249052
Relapsing-remitting multiple sclerosis as a potential consequence of thalidomide treatment: A case report., PMID:40245782
Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Current Management and Innovative Therapies., PMID:40231717
Risk of Serious Infections in Patients Treated With Biologic or Targeted-synthetic Disease Modifying Antirheumatic Drugs in Qatar., PMID:40230031
Drug- and Vaccine-Induced Cutaneous T-Cell Lymphoma: A Systematic Review of the Literature., PMID:40226161
Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis., PMID:40215252
Effects of biological agents on lipid profile and hemogram parameters in patients with psoriasis., PMID:40208364
Dual biological treatments in immune-mediated disorders: a single center experience., PMID:40200173
From the Cochrane Library: Anti-tumor necrosis factor agents for pediatric psoriasis., PMID:40187532
Optimizing Maternal and Fetal Antibody Exposure and Dosing Regimens During Pregnancy Using a Physiologically Based Pharmacokinetic Model., PMID:40181734
Analysis of the Reduction in the Duration of Sick Leave for 32,512 Psoriasis Patients Following the Integration of Targeted Therapies for Psoriatic Disease into the Brazilian Healthcare System: a Retrospective Cohort Study., PMID:40171531
Anti-TNFα as an Adjunctive Therapy in Pancreas and Kidney Transplantation., PMID:40170787
Mechanism and Efficacy of Etanercept in Treating Autoimmune-like Manifestations of Coronavirus Disease 2019 in elderly individuals., PMID:40168796
The Efficacy, Safety and Longevity of Biologic Treatments in Pediatric and Adult Psoriasis Patients: A Comparative Multi-Center, Real-Life Study., PMID:40165569