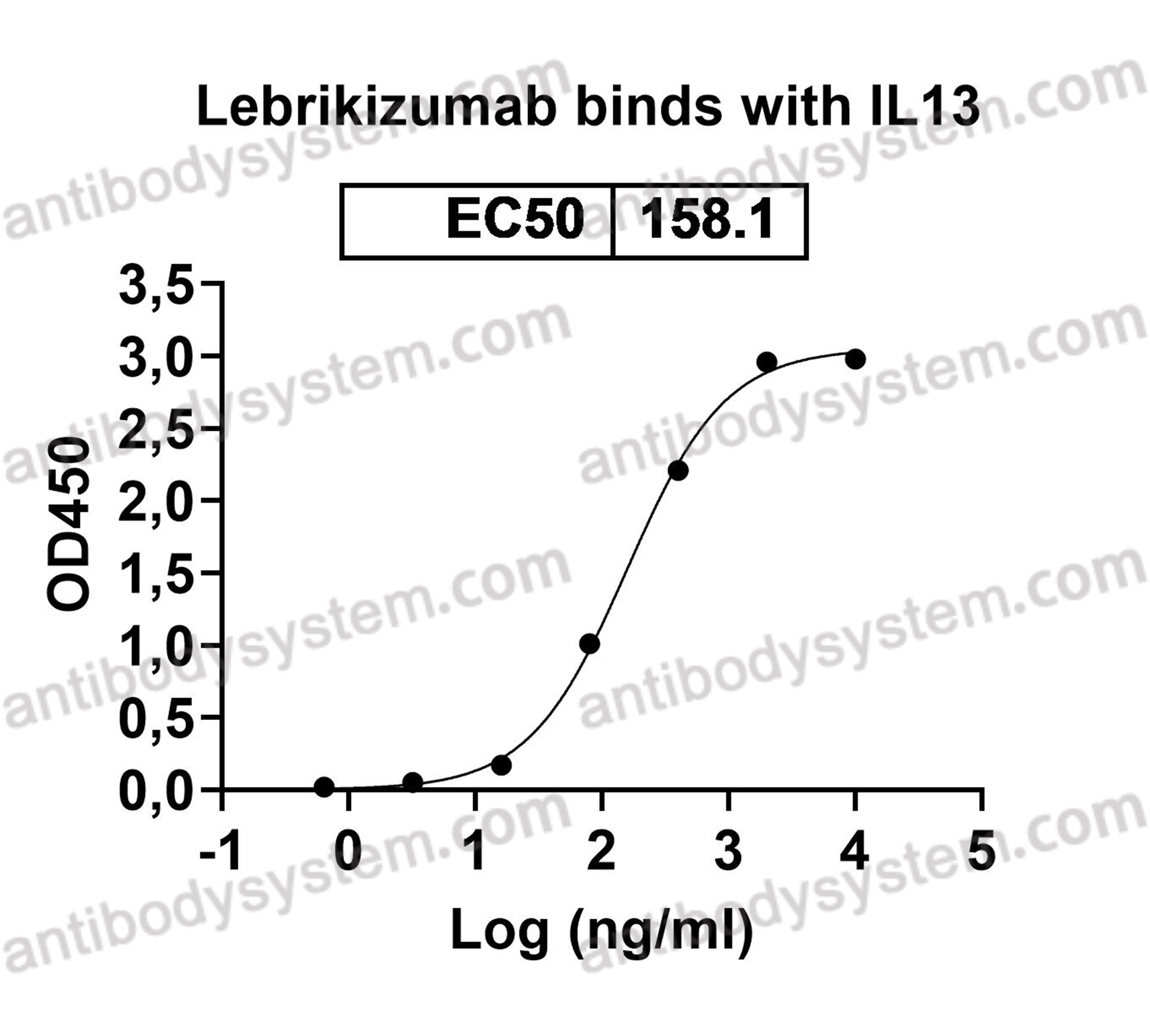
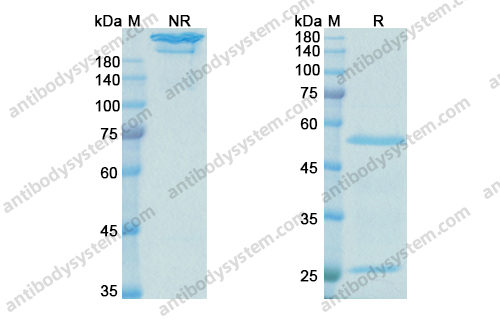
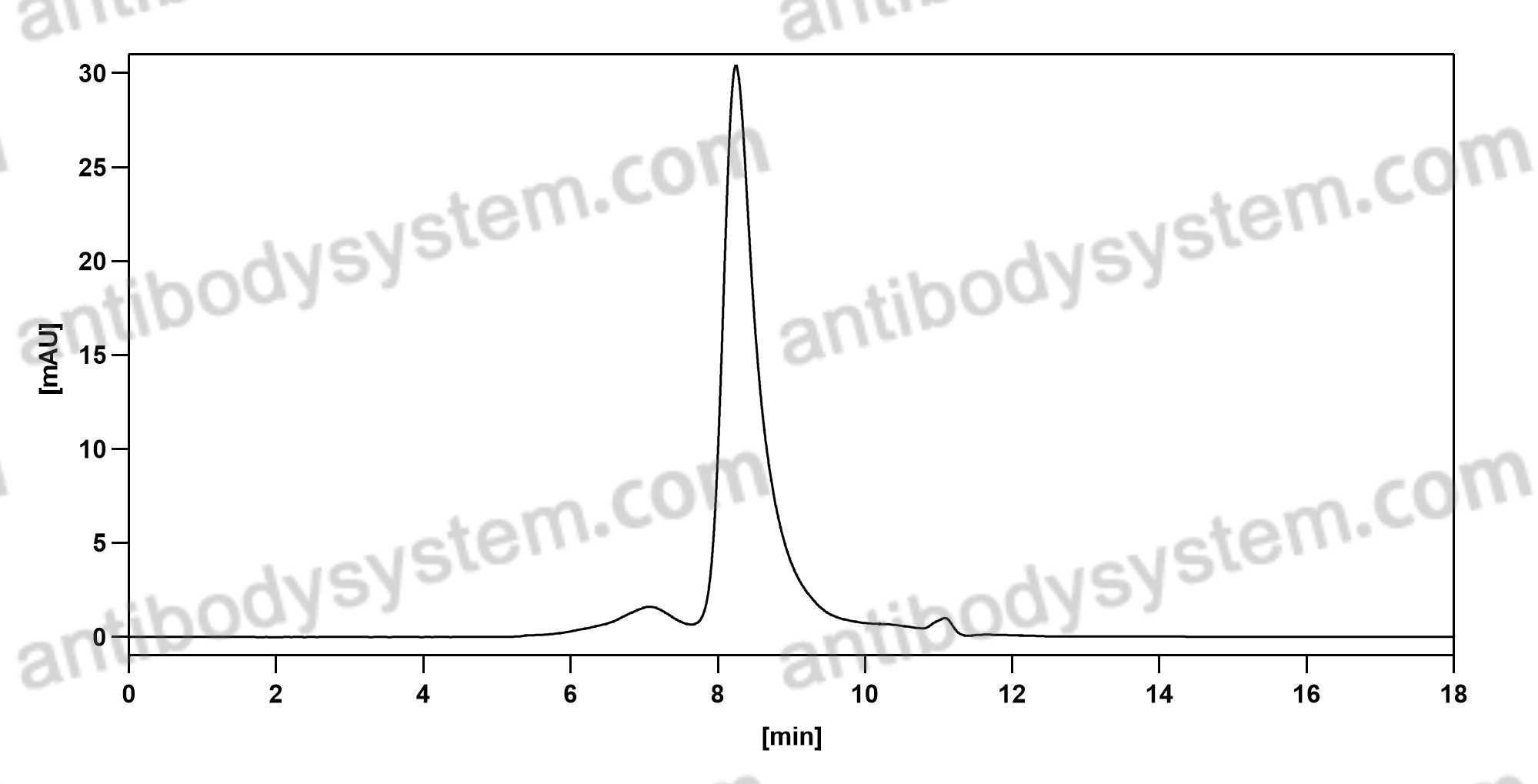
A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER), PMID: 32909660
Adult asthma biomarkers, PMID: 24300416
Are Biologics Efficacious in Atopic Dermatitis? A Systematic Review and Meta-Analysis, PMID: 29098604
Asthma phenotypes and endotypes, PMID: 23114560
Biological therapies for atopic dermatitis: An update, PMID: 30679974
Biologics for Atopic Dermatitis, PMID: 33012322
Clinical Applications Targeting Periostin, PMID: 31037637
Commonality of the IL-4/IL-13 pathway in atopic diseases, PMID: 28277826
Current and emerging biologics for the treatment of pediatric atopic dermatitis, PMID: 33078990
Docking analysis and the possibility of prediction efficacy for an anti-IL-13 biopharmaceutical treatment with tralokinumab and lebrikizumab for bronchial asthma, PMID: 29155876
Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids, PMID: 23726041
Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE), PMID: 29353026
Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids, PMID: 29413502
Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials, PMID: 27616196
Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial, PMID: 32101256
Efficacy of biologics in atopic dermatitis, PMID: 32003247
Emerging systemic therapies for atopic dermatitis: biologics, PMID: 33045848
Emerging Treatment Options in Atopic Dermatitis: Systemic Therapies, PMID: 29320765
Immunoglobulin interface redesigning to enhance lebrikizumab mediated immunomodulation of IL-13 hyper-response, PMID: 32448082
Innovation in Atopic Dermatitis: From Pathogenesis to Treatment, PMID: 31964499
Lebrikizumab for the treatment of asthma, PMID: 27554950
Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies, PMID: 26001563
Lebrikizumab in the personalized management of asthma, PMID: 23055690
Lebrikizumab in the treatment of asthma, PMID: 27161908
Lebrikizumab treatment in adults with asthma, PMID: 21812663
Lebrikizumab treatment in adults with asthma, PMID: 22187992
Lebrikizumab treatment in adults with asthma, PMID: 22187993
Lebrikizumab treatment in adults with asthma, PMID: 22187994
Meta-analysis of randomized controlled trials for the efficacy and safety of anti-interleukin-13 therapy with lebrikizumab in patients with uncontrolled asthma, PMID: 30153886
Model-based clinical pharmacology profiling and exposure-response relationships of the efficacy and biomarker of lebrikizumab in patients with moderate-to-severe asthma, PMID: 28843617
Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis, PMID: 31395084
New and Emerging Systemic Treatments for Atopic Dermatitis, PMID: 32519223
New and Emerging Therapies for Pediatric Atopic Dermatitis, PMID: 31364023
New Anti-Eosinophil Drugs for Asthma and COPD: Targeting the Trait!, PMID: 28583618
New perspectives of childhood asthma treatment with biologics, PMID: 30444939
Novel Therapeutic Approaches and Targets for the Treatment of Atopic Dermatitis, PMID: 32525769
Phase 2 trial to assess lebrikizumab in patients with idiopathic pulmonary fibrosis, PMID: 33008934
Profile of lebrikizumab and its potential in the treatment of asthma, PMID: 26309415
Relative efficacy of systemic treatments for atopic dermatitis, PMID: 30296535
Seasonal variability of severe asthma exacerbations and clinical benefit from lebrikizumab, PMID: 28238745
Selective IL-13 inhibitors for the treatment of atopic dermatitis, PMID: 33889195
Specific Immune Response to Phospholipase B-Like 2 Protein, a Host Cell Impurity in Lebrikizumab Clinical Material, PMID: 27739010
Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab, PMID: 23357170
Tailored therapy for severe asthma, PMID: 25671117
The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge, PMID: 24131304
The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma, PMID: 31866859
Therapeutic Potential of Lebrikizumab in the Treatment of Atopic Dermatitis, PMID: 32104006
Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches, PMID: 30825336
Update in asthma 2011, PMID: 22753688
What's New in Atopic Dermatitis, PMID: 30850043
Suppression of Il5 and Il13 Gene Expression by Synthetic siRNA Molecules Reduces Nasal Hyperreactivity and Inflammation in a Murine Model of Allergic Rhinitis., PMID:40451198
Clinical and Laboratory Indexes for Predicting Early and Late Responders to Lebrikizumab in Atopic Dermatitis: A Prospective Cohort Observational Study., PMID:40370216
Deciding Which Patients With Atopic Dermatitis to Prioritize for Biologics and Janus Kinase Inhibitors., PMID:40368249
European Guideline (EuroGuiDerm) on atopic eczema: Living update., PMID:40317496
A Case of Cutaneous Fungal Infection Following the Administration of Dupilumab., PMID:40291282
Incidence of upper respiratory tract infections with biological therapies in moderate to severe atopic dermatitis: a systematic review and meta-analysis., PMID:40241901
[Chronic Pruritus: A Review of Its Pathophysiology and Current Treatments]., PMID:40214004
Real-World Japanese Study of the Effectiveness and Safety of Switching From Dupilumab to Lebrikizumab in Patients with Moderate to Severe Atopic Dermatitis., PMID:40202458
Real-world experience of switching from tralokinumab to lebrikizumab in atopic dermatitis., PMID:40130939
24-Week Real-World Effectiveness and Safety of Lebrikizumab for Atopic Dermatitis in Japan: An Analysis Stratified by Prior Systemic Therapy., PMID:40098530
Investigating Efficacy of Atopic Dermatitis Systemic Therapeutics After Discontinuation Part I: Biologics., PMID:40078859
Performance characterization of spring-actuated prefilled pen devices for lebrikizumab and dupilumab., PMID:40053331
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
Real-World Effectiveness and Safety of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis: A 16-Week Study in Japan., PMID:39977111
Lebrikizumab vs Other Systemic Monotherapies for Moderate-to-Severe Atopic Dermatitis: Network Meta-analysis of Efficacy., PMID:39953372
Comparison chart: Interleukin (IL) receptor antagonists for atopic dermatitis., PMID:39946701
Nemolizumab (Nemluvio) for atopic dermatitis., PMID:39946696
Rosacea-like skin reaction under treatment with dupilumab for atopic dermatitis., PMID:39870387
Lebrikizumab decreases type 2 inflammatory biomarker levels in patients with asthma: data from randomized phase 3 trials (LAVOLTA I and II)., PMID:39781908
Lebrikizumab is associated with improvements in patient-reported symptoms and quality-of-life measures across Eczema Area and Severity Index response categories: pooled results from phase-3 randomized ADvocate1 and ADvocate2 studies in patients with moderate-to-severe atopic dermatitis., PMID:39761965
The efficacy of longer-term lebrikizumab treatment in patients with moderate-to-severe atopic dermatitis who did not meet protocol-defined response criteria at week 16 in 2 randomized controlled clinical trials., PMID:39733939
Advanced Biologic Therapies in the Management of Asthma in Children and Adolescents: A Comprehensive Network Meta-Analysis., PMID:39709955
Improved quality of life in patients treated with lebrikizumab monotherapy is mediated by improvements in itch and sleep: results from two phase III trials in patients with moderate-to-severe atopic dermatitis., PMID:39680511
Lebrikizumab: a new anti-IL-13 agent for treating moderate-to-severe atopic dermatitis., PMID:39641601
Evaluation of Pharmacokinetics of Lebrikizumab in Healthy Individuals After Subcutaneous Administration Using a Prefilled Syringe or Autoinjector in a Phase 1 Randomized Study., PMID:39638723
Correction to: Improvement Across Dimensions of Disease with Lebrikizumab Use in Atopic Dermatitis: Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Monotherapy Trials (ADvocate1 and ADvocate2)., PMID:39636571
Efficacy and safety of lebrikizumab for the treatment of moderate-to-severe atopic dermatitis: a systematic review and meta-analysis., PMID:39629076
Efficacy and safety of lebrikizumab combined with topical corticosteroids in Japanese patients with moderate-to-severe atopic dermatitis: a phase 3, double-blind, placebo-controlled, randomized clinical trial (ADhere-J)., PMID:39625230
Biologics in allergology and clinical immunology: Update on therapies for atopic diseases, urticaria, and angioedema and on safety aspects focusing on hypersensitivity reactions., PMID:39600395
Atopic Dermatitis-Related Problems in Daily Life, Goals of Therapy and Deciding Factors for Systemic Therapy: A Review., PMID:39598368
Comparative efficacy of targeted systemic therapies for pruritus in moderate-to-severe atopic dermatitis without topical treatment: a network meta-analysis., PMID:39592133
Lebrikizumab-lbkz., PMID:39561252
IL-13 inhibition in the treatment of atopic dermatitis - new and emerging biologic agents., PMID:39558725
[Not Available]., PMID:39551509
Lebrikizumab (Ebglyss) for atopic dermatitis., PMID:39509156
The Pathophysiology, Diagnosis and Management of Chronic Inflammatory Skin Diseases., PMID:39463216
Long-term management of moderate-to-severe atopic dermatitis with lebrikizumab and concomitant topical corticosteroids: a 68-week randomized double-blind placebo-controlled phase III trial in Japan (ADhere-J)., PMID:39442013
Publisher Correction: Patients with Moderate-to-Severe Atopic Dermatitis Maintain Stable Response with No or Minimal Fluctuations with 1 Year of Lebrikizumab Treatment., PMID:39424713
Current and Emerging Biologics for Atopic Dermatitis., PMID:39389711
Lebrikizumab approval for the treatment of moderate to severe atopic dermatitis in Italy., PMID:39325369
Improvement Across Dimensions of Disease with Lebrikizumab Use in Atopic Dermatitis: Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Monotherapy Trials (ADvocate1 and ADvocate2)., PMID:39249591
Lebrikizumab Combined with Topical Corticosteroids Improves Patient-reported Outcomes in Japanese Patients with Moderate-to-severe Atopic Dermatitis., PMID:39248292
An expert consensus on managing dupilumab-related ocular surface disorders in people with atopic dermatitis 2024., PMID:39236226
Biologic and Small Molecule Therapy in Atopic Dermatitis., PMID:39200305
Extended Half-life Antibodies: A Narrative Review of a New Approach in the Management of Atopic Dermatitis., PMID:39147994
Patients with Moderate-to-Severe Atopic Dermatitis Maintain Stable Response with No or Minimal Fluctuations with 1 Year of Lebrikizumab Treatment., PMID:39123054
Safety and efficacy of biologic drugs in children or adolescents with atopic dermatitis: A systematic review and meta-analysis of randomized controlled trials., PMID:39101303