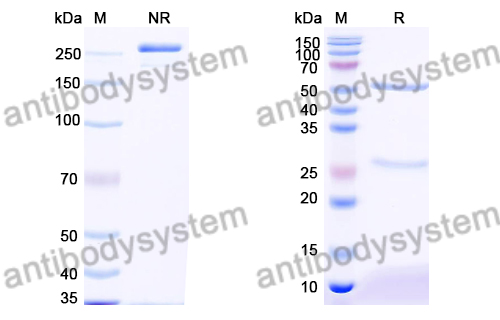
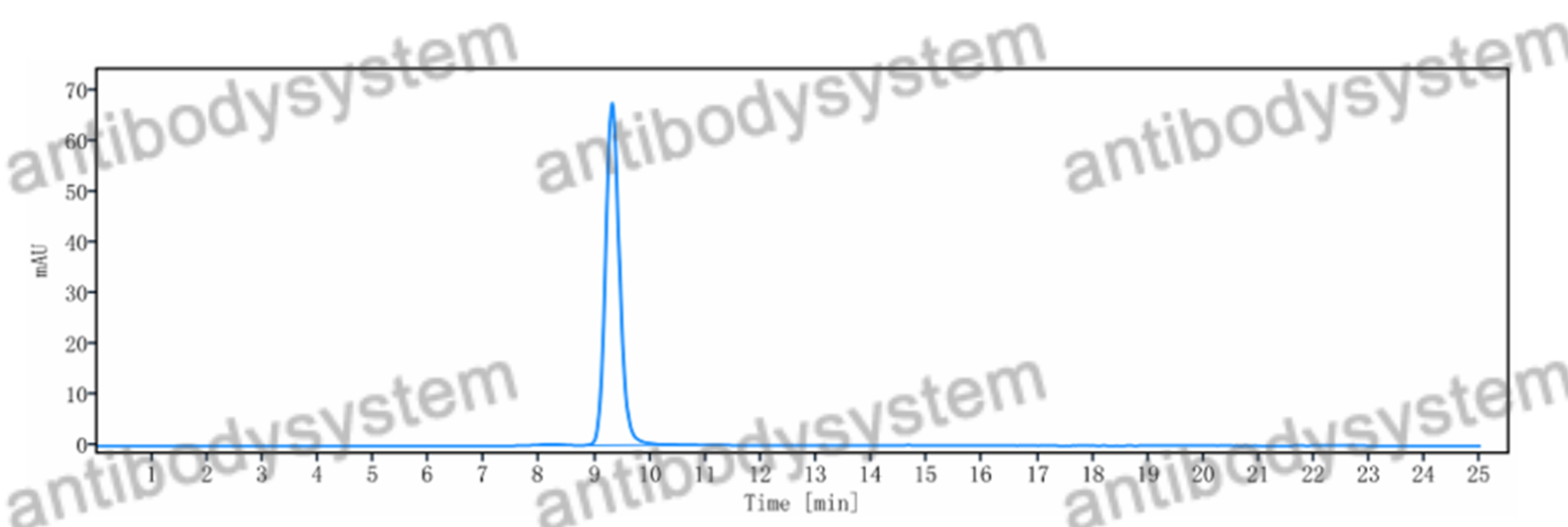
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of belantamab mafodotin for multiple myeloma, asciminib for leukemia, osimertinib for lung cancer, amivantamab for lung cancer, and pembrolizumab for pleural mesothelioma in Japan., PMID:40481943
Real-world characteristics and outcomes of patients with multiple myeloma treated with belantamab mafodotin: a German claims data study., PMID:40448822
Population Pharmacokinetics for Belantamab Mafodotin Monotherapy and Combination Therapies in Patients with Relapsed/Refractory Multiple Myeloma., PMID:40447948
Practice-changing updates on multiple myeloma: highlights from the 2024 ASH annual meeting., PMID:40361170
Treatment Patterns, Efficacy, and Tolerability of Belantamab Mafodotin in Patients With Relapsed and/or Refractory Multiple Myeloma: A Real-World Analysis., PMID:40348718
Characterization of Belantamab Mafodotin-Induced Corneal Changes in Patients With Multiple Myeloma., PMID:40338596
Corneal Findings in Patients Treated with Belantamab Mafodotin: A Prospective Case Series Focusing on Corneal Nerves., PMID:40327294
Belantamab Mafodotin Monotherapy for Multiply-Relapsed Myeloma: A Retrospective Study From the United Kingdom and the Republic of Ireland., PMID:40308262
HSR25-172: Meta-Analysis of Phase 3 Randomized Controlled Trials to Evaluate the Incidence of Infectious Adverse Events in Patients With Relapsed/Refractory Multiple Myeloma Treated With Belantamab Mafodotin., PMID:40154473
HSR25-143: Meta-Analysis of Phase 3 Randomized Controlled Trials to Evaluate the Incidence of Systemic Toxicities in Patients With Relapsed/Refractory Multiple Myeloma Treated With Belantamab Mafodotin., PMID:40154424
Belantamab Mafodotin Plus Proteasome Inhibition Efficacy Versus Comparators in Early Relapsed Myeloma: A Systematic Review and Network Meta-Analysis., PMID:40143674
Correction: The clinical journey of belantamab mafodotin in relapsed or refractory multiple myeloma: lessons in drug development., PMID:40140348
Current Treatment Strategies for Multiple Myeloma at First Relapse., PMID:40095642
Unveiling cardiovascular and respiratory toxicities with monoclonal antibodies in multiple myeloma: disproportionality analysis from the FDA Adverse Event Reporting System., PMID:40095047
Novelties on Multiple Myeloma from the Main 2024 Hematology Conferences., PMID:40084104
A real-world experience of efficacy and safety of belantamab mafodotin in relapsed refractory multiple myeloma., PMID:40064854
Prior exposure to belantamab mafodotin influences outcomes with idecabtagene vicleucel in patients with multiple myeloma., PMID:39938011
The clinical journey of belantamab mafodotin in relapsed or refractory multiple myeloma: lessons in drug development., PMID:39920159
Novel Treatment Options for Multiple Myeloma., PMID:39772633
Update on B-cell maturation antigen-directed therapies in AL amyloidosis., PMID:39748220
DREAMM-11, Part 2: Japanese phase I trial of belantamab mafodotin combination therapies in relapsed/refractory multiple myeloma., PMID:39718747
The Antibody Drug Conjugate, Belantamab-Mafodotin, in the Treatment of Multiple Myeloma: A Comprehensive Review., PMID:39664714
Focal and Segmental Glomerulosclerosis in a Multiple Myeloma Patient After Belantamab Mafodotin Therapy and Severe COVID-19 Infection: A Case Report., PMID:39482831
Neuropsychiatric Adverse Events with Monoclonal Antibodies Approved for Multiple Myeloma: An Analysis from the FDA Adverse Event Reporting System., PMID:39458907
Strengths and Weaknesses of Different Therapeutic Strategies for the Treatment of Patients with Multiple Myeloma Who Progress After the Frontline Use of Lenalidomide: A Narrative Review., PMID:39458188
Results from Arm A of Phase 1/2 DREAMM-6 trial: belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma., PMID:39433730
Belantamab mafodotin monotherapy for relapsed or refractory multiple myeloma: a real-world observational study in the United States., PMID:39415693
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma. Reply., PMID:39383466
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma., PMID:39383465
Belantamab Mafodotin in Relapsed/Refractory AL Amyloidosis: Real-World Multi-Center Experience and Review of the Literature., PMID:39357511
Preclinical Evaluation of STI-8811, a Novel Antibody-Drug Conjugate Targeting BCMA for the Treatment of Multiple Myeloma., PMID:39292169
Drug-Related Keratitis: A Real-World FDA Adverse Event Reporting System Database Study., PMID:39287587
How to rescue a DreaMM deferred …., PMID:39276768
Belantamab mafodotin, pomalidomide, and dexamethasone for triple class exposed/refractory relapsed multiple myeloma: a subgroup analysis of the ALGONQUIN trial., PMID:39261451
Ocular adverse events associated with antibody-drug conjugates in oncology: a pharmacovigilance study based on FDA adverse event reporting system (FAERS)., PMID:39228525
Matching-adjusted indirect comparison of talquetamab vs selinexor-dexamethasone and vs belantamab mafodotin in patients with relapsed/refractory multiple myeloma., PMID:39226081
The Role of Monoclonal Antibodies in the Treatment of Myeloma Kidney Disease., PMID:39204135
Dynamics of microcyst-like epithelial changes associated with Belantamab mafodotin therapy in a patient with multiple myeloma-a case report., PMID:39112495
A Belantamab Mafodotin Revival in Multiple Myeloma Therapy., PMID:39083777
Belantamab-mafodotin-associated keratopathy., PMID:39003154
Corneal Toxicity in Patients Treated by BELANTAMAB MAFODOTIN: How to Improve and Facilitate Patients Follow-Up Using Refractive Shift?, PMID:38976493
Belantamab mafodotin concentration-QTc relationships in patients with relapsed or refractory multiple myeloma from the DREAMM-1 and -2 studies., PMID:38924122
Visualization of Keratopathy Associated With the Antibody-Drug Conjugate Belantamab Mafodotin Using Infrared Imaging in Patients With Multiple Myeloma., PMID:38900711
Belantamab mafodotin in triple-refractory multiple myeloma patients: A retro-prospective observational study in Italy., PMID:38895069
Successful treatment with belantamab mafodotin in a heavily pretreated patient with multiple myeloma and liver extramedullary disease., PMID:38850573
Belantamab Mafodotin, Pomalidomide, and Dexamethasone in Multiple Myeloma., PMID:38828951
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma., PMID:38828933
Clinical evaluation and determinants of response to HBI0101 (BCMA CART) therapy in relapsed/refractory multiple myeloma., PMID:38768428
Antigen escape as a shared mechanism of resistance to BCMA-directed therapies in multiple myeloma., PMID:38728378
Effectiveness and safety of belantamab mafodotin in patients with relapsed or refractory multiple myeloma in real-world setting: The ALFA study., PMID:38722078