Catalog No.
KAD12652
Description
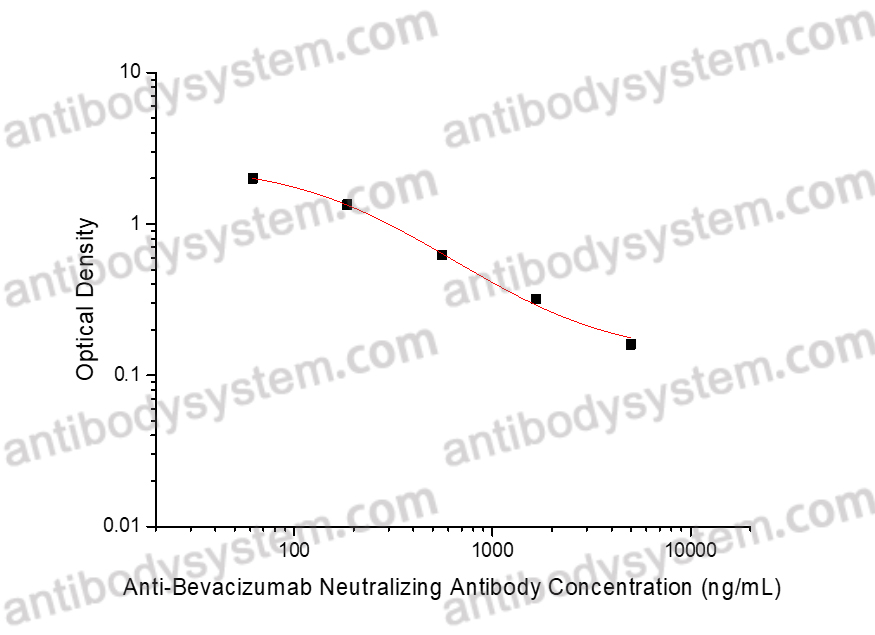
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Bevacizumab has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled Human VEGFA antigen and then pipetted into the wells. Anti-Bevacizumab Neutralizing Antibody in the sample competitively binds to Bevacizumab with the biotin-labeled Human VEGFA antigen. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Anti-Bevacizumab Neutralizing Antibody bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Bevacizumab Neutralizing Antibody concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
61.73 - 5,000 ng/mL
Sensitivity
8.26 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2517.8
|
898.7
|
141.2
|
2816.2
|
870.1
|
167.2
|
|
Standard deviation
|
318.1
|
92.2
|
14.5
|
483.0
|
92.8
|
28.8
|
|
CV (%)
|
12.6
|
10.3
|
10.3
|
17.2
|
10.7
|
17.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
12-IgG1,F(ab)-12 IgG1,Fab-12 IgG1,rhuMAb-VEGF, ABP 215, CAS: 216974-75-3
An endothelial cell competition assay for determinants of the response to targeted anti-angiogenics., PMID:40526406
Addition of Bevacizumab to Vinorelbine-Platinum combination is efficacious in Heavily Pretreated HER2-Negative Metastatic Breast Cancer., PMID:39991566
Safety and efficacy of trifluridine/tipiracil +/- bevacizumab plus XB2001 (anti-IL-1α antibody): a single-center phase 1 trial., PMID:39820336
VEGF-Virus Interactions: Pathogenic Mechanisms and Therapeutic Applications., PMID:39513922
Role of transforming growth factor-β1 pathway in angiogenesis induced by chronic stress in colorectal cancer., PMID:38857055
Comparison of Efficacy and Safety of a Bevacizumab Biosimilar, in Combination with Chemotherapies, in Nonresectable Metastatic Colorectal Cancer and in Advanced Nonsquamous Non-Small Cell Lung Cancer: A Randomized, Double-Blind, Phase III Study., PMID:38721097
Targeting VEGF using Bevacizumab attenuates sepsis-induced liver injury in a mouse model of cecal ligation and puncture., PMID:38313162
A2AR-mediated lymphangiogenesis via VEGFR2 signaling prevents salt-sensitive hypertension., PMID:37377160
Tumor steatosis and glutamine synthetase expression in patients with advanced hepatocellular carcinoma receiving atezolizumab plus bevacizumab therapy., PMID:37300323
A Randomized, Double-Blind, Parallel-Controlled Phase I Study Comparing the Pharmacokinetics, Safety, and Immunogenicity of SCT510 to Bevacizumab (Avastin®) in Healthy Chinese Males., PMID:37247166
A randomized, double-blind, single-dose study to assess bioequivalence of MB02 biosimilar after manufacturing iteration and reference bevacizumab., PMID:36914963
L1CAM promotes vasculogenic mimicry formation by miR-143-3p-induced expression of hexokinase 2 in glioma., PMID:36708044
Circulating galectin-1 delineates response to bevacizumab in melanoma patients and reprograms endothelial cell biology., PMID:36634146
Bevacizumab biosimilar candidate TAB008 compared to Avastin® in patients with locally advanced, metastatic EGFR wild-type non-squamous non-small cell lung cancer: a randomized, double-blind, multicenter study., PMID:36595042
Endothelial-Specific Molecule 1 Inhibition Lessens Productive Angiogenesis and Tumor Metastasis to Overcome Bevacizumab Resistance., PMID:36428773
Arteriovenous thrombotic events in a patient with advanced lung cancer following bevacizumab plus chemotherapy: A case report., PMID:35979297
Emerging immunotherapy for HCC: A guide for hepatologists., PMID:35253934
Pharmacokinetics, Tolerability, Safety, and Immunogenicity of LY01008 and Bevacizumab (Avastin®) in Healthy Chinese Subjects., PMID:35112328
Interactions Between Anti-Angiogenic Therapy and Immunotherapy in Glioblastoma., PMID:35096619
Schwannoma Gene Therapy via Adeno-Associated Viral Vector Delivery of Apoptosis-Associated Speck-like Protein Containing CARD (ASC): Preclinical Efficacy and Safety., PMID:35055004
Phase III double-blind study comparing the efficacy and safety of proposed biosimilar MYL-1402O and reference bevacizumab in stage IV non-small-cell lung cancer., PMID:34819997
Receptor tyrosine kinases as druggable targets in glioblastoma: Do signaling pathways matter?, PMID:34806012
Intravitreal connective tissue growth factor neutralizing antibody or bevacizumab alone or in combination for prevention of proliferative vitreoretinopathy in an experimental model., PMID:34022176
Efficacy, Safety and Immunogenicity of MB02 (Bevacizumab Biosimilar) versus Reference Bevacizumab in Advanced Non-Small Cell Lung Cancer: A Randomized, Double-Blind, Phase III Study (STELLA)., PMID:33914256
Advances in immunotherapy for hepatocellular carcinoma., PMID:33850328
HEYL Regulates Neoangiogenesis Through Overexpression in Both Breast Tumor Epithelium and Endothelium., PMID:33520697
Production of the Cytokine VEGF-A by CD4+ T and Myeloid Cells Disrupts the Corneal Nerve Landscape and Promotes Herpes Stromal Keratitis., PMID:33207210
Isolation of anti-VEGF monoclonal antibodies with neutralizing effects from an Astragalus-induced immune antibody library., PMID:33182041
Quantification of Bevacizumab Activity Following Treatment of Patients With Ovarian Cancer or Glioblastoma., PMID:33178180
A phase I, randomized, single-dose pharmacokinetic study comparing sb8 (bevacizumab biosimilar) with reference bevacizumab in healthy volunteers., PMID:32949267
Vascular endothelial growth factor contributes to lung vascular hyperpermeability in sepsis-associated acute lung injury., PMID:32696151
The immune-checkpoint HLA-G/ILT4 is involved in the regulation of VEGF expression in clear cell renal cell carcinoma., PMID:32620162
Development and characterization of a fully human antibody targeting SCF/c-kit signaling., PMID:32437800
Resistance Mechanisms to Anti-angiogenic Therapies in Cancer., PMID:32175278
Rewiring of Lipid Metabolism and Storage in Ovarian Cancer Cells after Anti-VEGF Therapy., PMID:31835444
Comparing The Efficacy Of An Anti-Human VEGF-A Neutralizing Antibody Versus Bevacizumab On A Laser-Induced Choroidal Neovascularization (CNV) Rhesus Monkey Model., PMID:31806932
Systemic drugs with impact on osteoarthritis., PMID:31726891
Effect of bevacizumab on the tight junction proteins of vascular endothelial cells., PMID:31632528
A Phase I, Randomized, Single-Dose Study Evaluating the Biosimilarity of TAB008 to Bevacizumab in Healthy Volunteers., PMID:31474863
Effects of intravitreal connective tissue growth factor neutralizing antibody on choroidal neovascular membrane-associated subretinal fibrosis., PMID:31029789
Efficacy and Safety of the Biosimilar ABP 215 Compared with Bevacizumab in Patients with Advanced Nonsquamous Non-small Cell Lung Cancer (MAPLE): A Randomized, Double-blind, Phase III Study., PMID:30617139
Nuclear magnetic resonance metabolic fingerprint of bevacizumab in mutant IDH1 glioma cells., PMID:30511933
Three-Dimensional Transport Model for Intravitreal and Suprachoroidal Drug Injection., PMID:30383198
Selective Inhibition of ADAM28 Suppresses Lung Carcinoma Cell Growth and Metastasis., PMID:30190423
Tolerance, variability, and pharmacokinetics of bevacizumab biosimilars in Chinese healthy male subjects., PMID:30043208
Modulation of Circulating Protein Biomarkers in Cancer Patients Receiving Bevacizumab and the Anti-Endoglin Antibody, TRC105., PMID:29997150
Anti-angiogenic treatment (Bevacizumab) improves the responsiveness of photodynamic therapy in colorectal cancer., PMID:29894822
A predictive value of von Willebrand factor for early response to Bevacizumab therapy in recurrent glioma., PMID:29594657
A safety and immunogenicity study of immunization with hVEGF26-104/RFASE in cynomolgus monkeys., PMID:29519591
Cross-activating c-Met/β1 integrin complex drives metastasis and invasive resistance in cancer., PMID:28973887