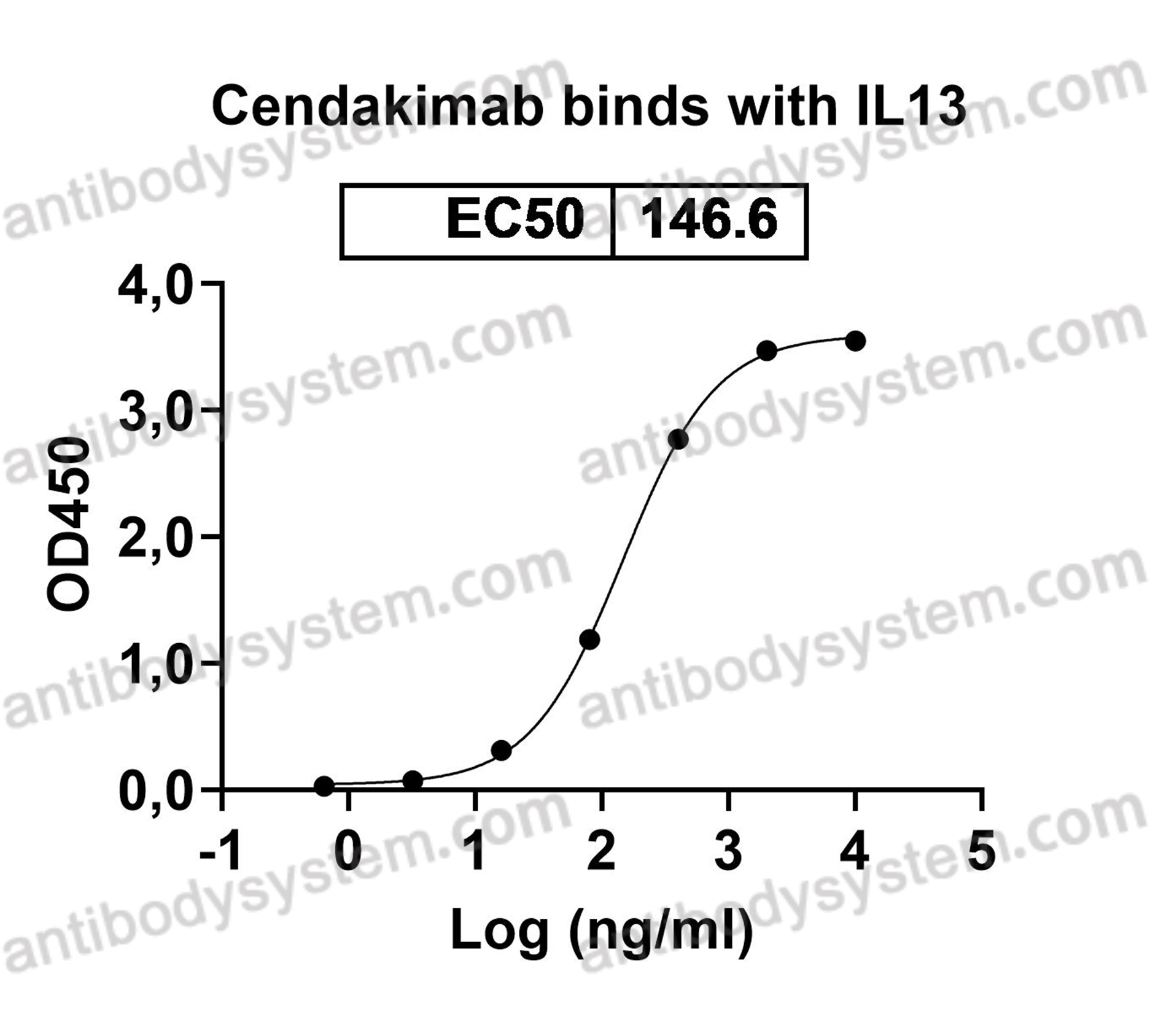
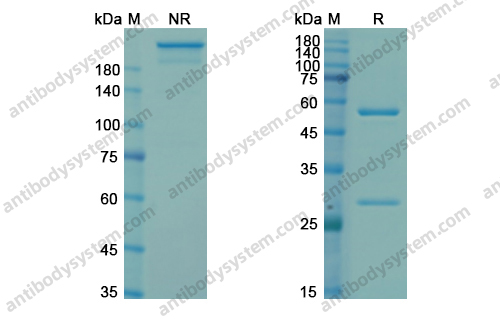
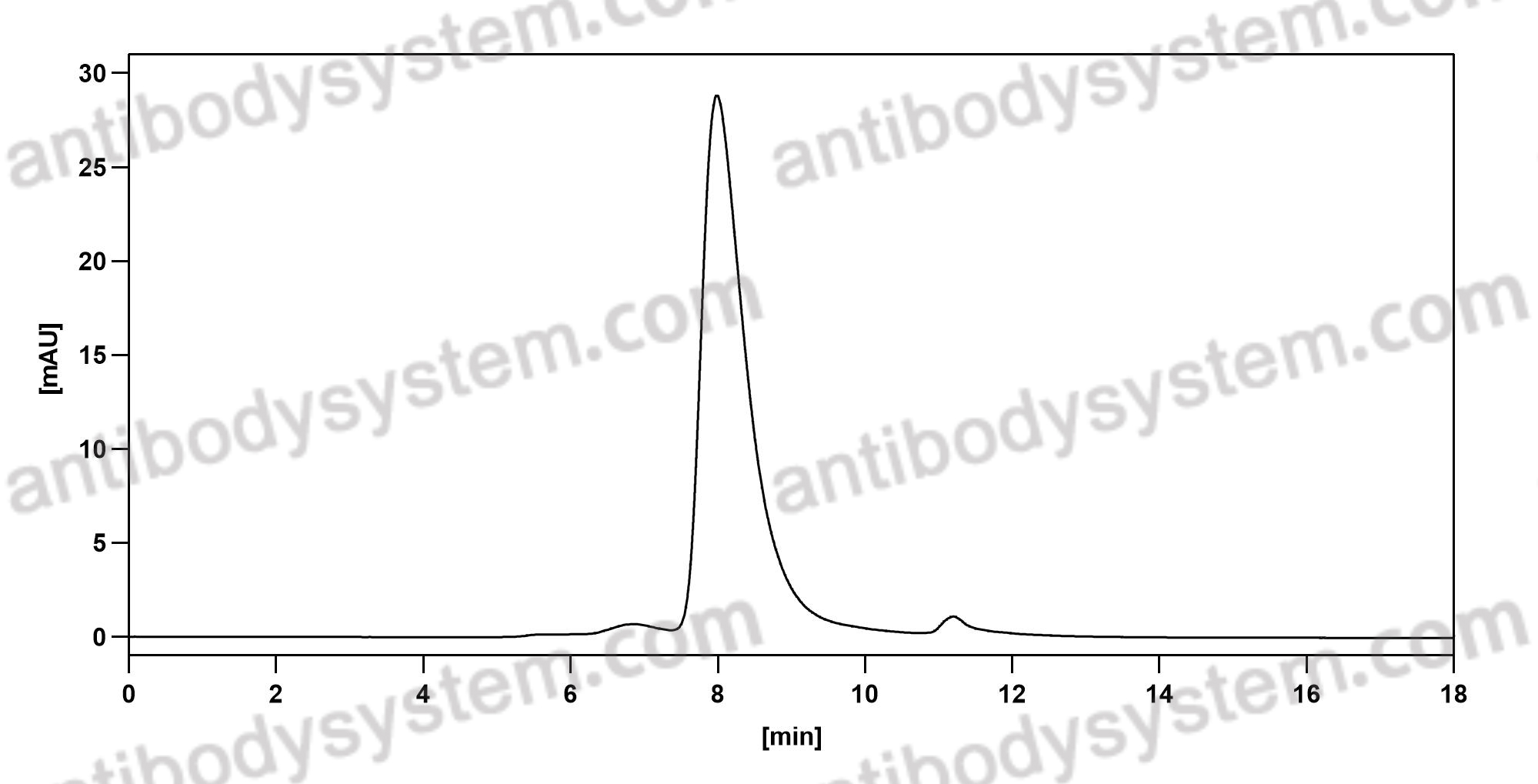
Catalog No.
DHE07701
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
IL13, NC30, Interleukin-13, IL-13
Concentration
5.25 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P35225
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
13C5.5, ABT-308, RPC-4046, CAS: 2151032-62-9
Clone ID
Cendakimab
Long-term Efficacy and Tolerability of RPC4046 in an Open-Label Extension Trial of Patients With Eosinophilic Esophagitis, PMID: 32205221
One If By Steroids and Two If By Biologic, PMID: 30641052
RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis, PMID: 30395812
RPC4046, A Novel Anti-interleukin-13 Antibody, Blocks IL-13 Binding to IL-13 α1 and α2 Receptors: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation First-in-Human Study, PMID: 28455782
Targeted Therapies for Eosinophilic Gastrointestinal Disorders, PMID: 32472465
Pharmacokinetic Characterization of Cendakimab Administered with Different Devices and at Different Injection Sites in Healthy Participants., PMID:40374841
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
IL-13 inhibition in the treatment of atopic dermatitis - new and emerging biologic agents., PMID:39558725
Design of a phase 3, randomized, double-blind, placebo-controlled, 48-week study to evaluate the efficacy and safety of cendakimab in adult and adolescent patients with eosinophilic esophagitis., PMID:39384067
Cendakimab in Patients With Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial., PMID:39018038
Histological Outcomes of Pharmacological Interventions in Eosinophilic Esophagitis for Adults and Children: A Network Meta-analysis of Randomized Controlled Trials., PMID:38701235
The New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis: Biological Drugs., PMID:38338983
Binding, Neutralization and Internalization of the Interleukin-13 Antibody, Lebrikizumab., PMID:37310643
Biologic Therapies Targeting Eosinophilic Gastrointestinal Diseases., PMID:37081678
Emerging therapies for eosinophilic esophagitis., PMID:36840634
Targeting Interleukin 13 for the Treatment of Atopic Dermatitis., PMID:36839890
Dysphagia Days as an Assessment of Clinical Treatment Outcome in Eosinophilic Esophagitis., PMID:36647838
Biologics in eosinophilic gastrointestinal diseases., PMID:35738437
Update on Emerging Pharmacologic Therapies for Patients With Eosinophilic Esophagitis., PMID:35505944
Drug treatment strategies for eosinophilic esophagitis in adults., PMID:35379069
Current options and investigational drugs for the treatment of eosinophilic esophagitis., PMID:35072575
Targeted Therapies for Eosinophilic Gastrointestinal Disorders., PMID:32472465
Long-term Efficacy and Tolerability of RPC4046 in an Open-Label Extension Trial of Patients With Eosinophilic Esophagitis., PMID:32205221
One If By Steroids and Two If By Biologic., PMID:30641052
RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis., PMID:30395812
RPC4046, A Novel Anti-interleukin-13 Antibody, Blocks IL-13 Binding to IL-13 α1 and α2 Receptors: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation First-in-Human Study., PMID:28455782