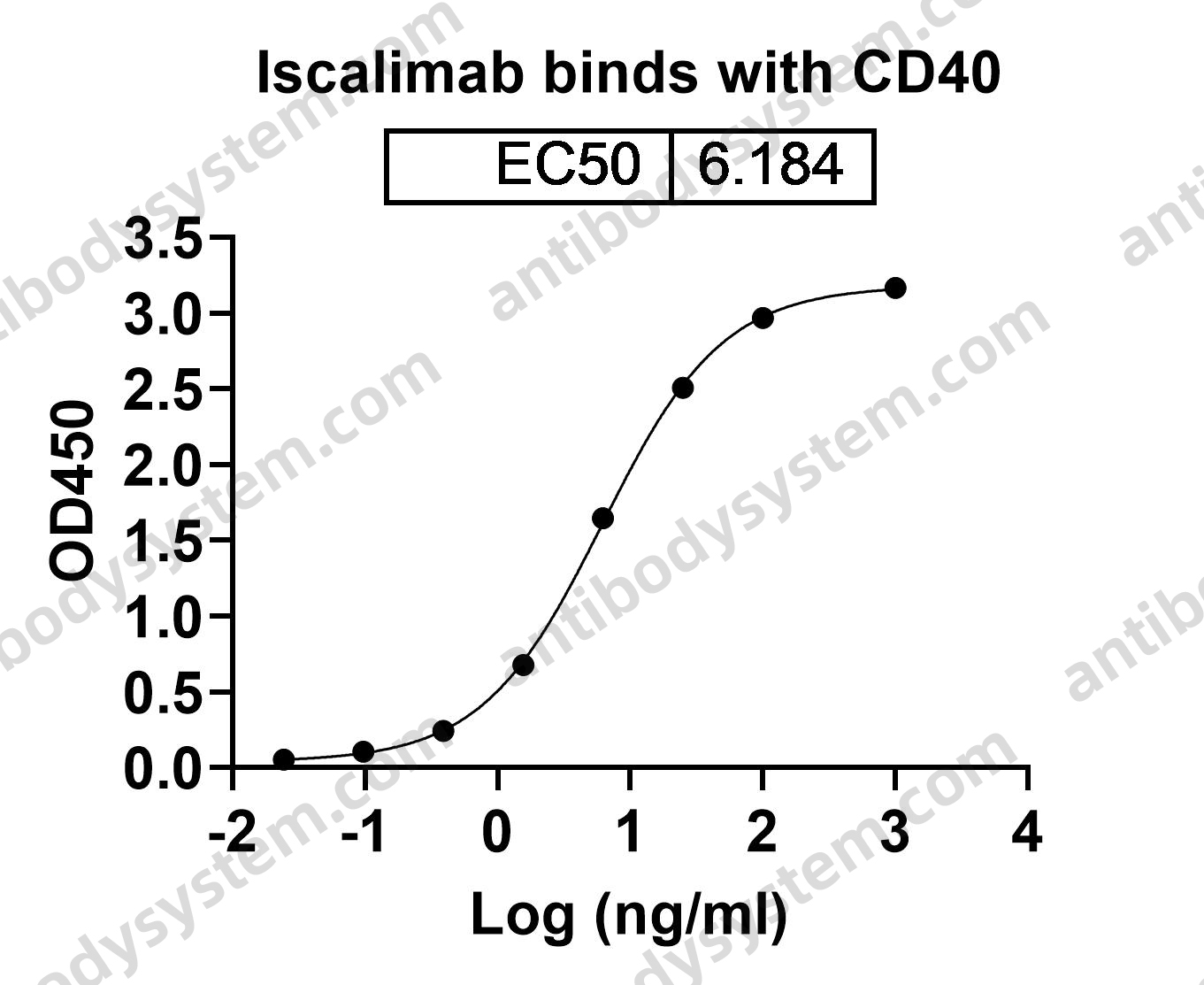
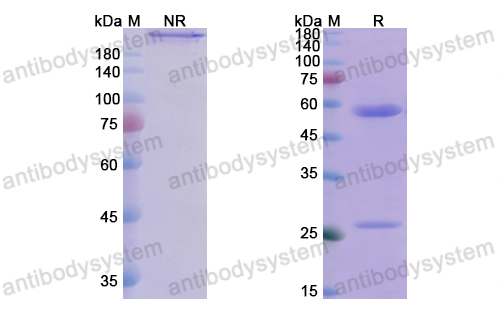
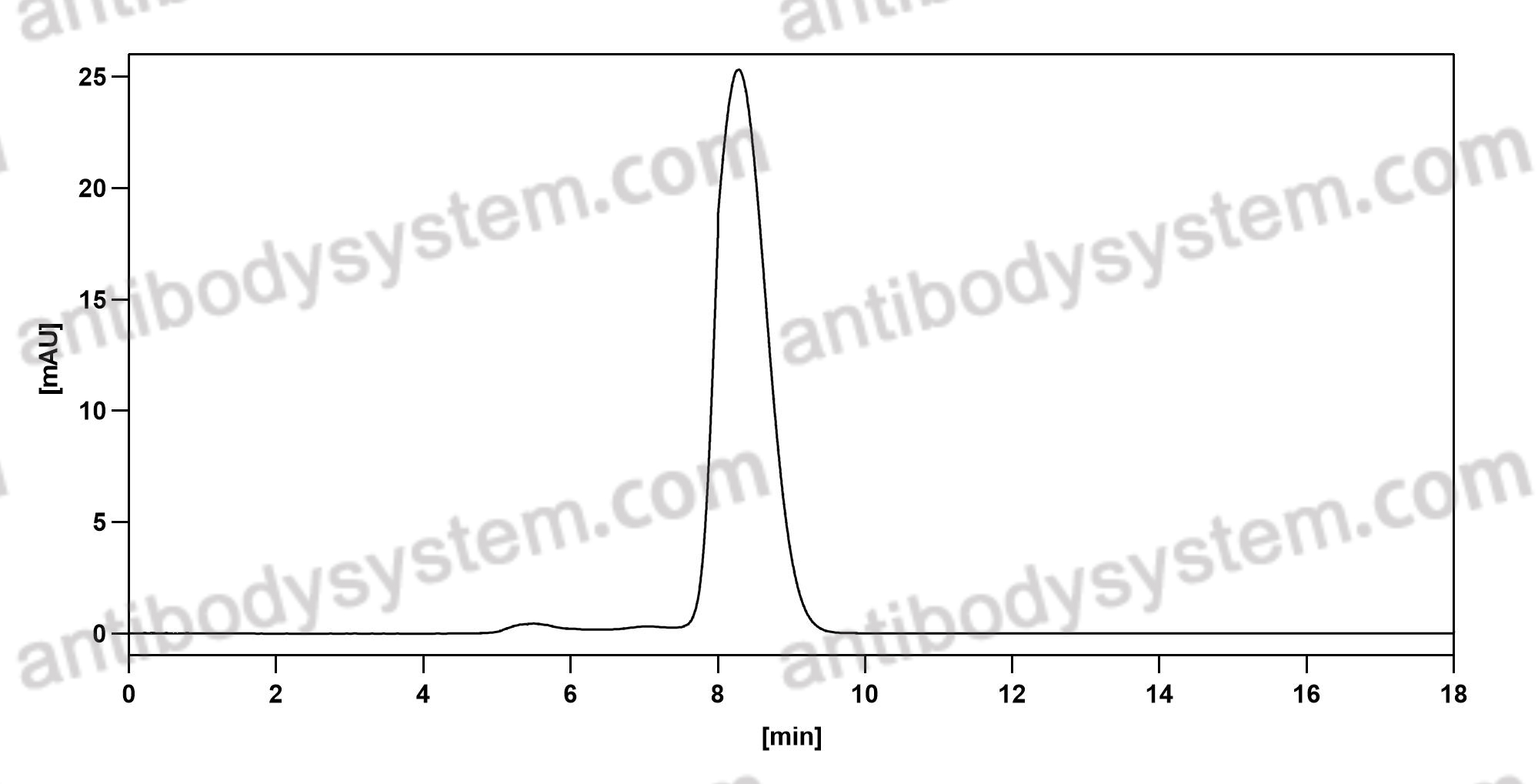
Catalog No.
DHD68901
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Tumor necrosis factor receptor superfamily member 5, B-cell surface antigen CD40, Bp50, CD40L receptor, TNFRSF5, CDw40, CD40
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P25942
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CFZ-533, NVP-CFZ533, OM11-62MF, CAS: 2031153-61-2
Clone ID
Iscalimab
First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody, PMID: 31647605
A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial, PMID: 31512728
Sjögren's syndrome: Old and new therapeutic targets, PMID: 31831255
Biologics in the treatment of Sjogren's syndrome, systemic lupus erythematosus, and lupus nephritis, PMID: 33002950
Blocking T cell co-stimulation in primary Sjögren's syndrome: rationale, clinical efficacy and modulation of peripheral and salivary gland biomarkers, PMID: 33095146
Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody, PMID: 29665205
Developments in immunosuppression, PMID: 33332922
Precision Medicine in Graves' Disease: CD40 Gene Variants Predict Clinical Response to an Anti-CD40 Monoclonal Antibody, PMID: 34149627
[Sjögren's syndrome: from diagnosis to treatment], PMID: 33689243
Novel Approaches for Immunosuppression in Graves' Hyperthyroidism and Associated Orbitopathy, PMID: 33511082
Current status of costimulatory blockade in renal transplantation, PMID: 27517137
Nonclinical Safety Assessment of CFZ533, a Fc-Silent Anti-CD40 Antibody, in Cynomolgus Monkeys, PMID: 30099540
Iscalimab 600 mg: justified or excessive?, PMID:40318875
Iscalimab 600 mg: justified or excessive? - Authors' reply., PMID:40318874
Targeted immunotherapies for Graves' thyroidal & orbital diseases., PMID:40145088
Costimulation blockade: the next generation., PMID:39882641
[Iscalimab-New treatment option for patients with primary Sjögren's disease]., PMID:39738525
Infections in Sjögren's disease: a clinical concern or not?, PMID:39661562
New Developments and Therapeutic Drug Monitoring Options in Costimulatory Blockade in Solid Organ Transplantation: A Systematic Critical Review., PMID:39570574
Iscalimab Combined With Transient Tesidolumab Prolongs Survival in Pig-to-Rhesus Monkey Renal Xenografts., PMID:39185772
Safety and efficacy of subcutaneous iscalimab (CFZ533) in two distinct populations of patients with Sjögren's disease (TWINSS): week 24 results of a randomised, double-blind, placebo-controlled, phase 2b dose-ranging study., PMID:39096929
[Focus on Sjögren's syndrome - Diagnosis and treatment]., PMID:38781999
Efficacy and safety of iscalimab, a novel anti-CD40 monoclonal antibody, in moderate-to-severe myasthenia gravis: A phase 2 randomized study., PMID:37988976
Concurrent Ocular and Cerebral Toxoplasmosis in a Liver Transplant Patient Treated with Anti-CD40 Monoclonal Antibody., PMID:37545749
Single-cell transcriptomic analysis of renal allograft rejection reveals insights into intragraft TCR clonality., PMID:37227784
Single cell transcriptomic analysis of renal allograft rejection reveals novel insights into intragraft TCR clonality., PMID:36798151
Non-thionamide antithyroid drug options in Graves' hyperthyroidism., PMID:36740774
Tuberculosis dissemination in kidney transplant recipient treated with anti-CD40 monoclonal antibody: a case report., PMID:35986231
Immunosuppression Profile of CFZ533 (Iscalimab), a Non-Depleting Anti-CD40 Antibody, and the Presence of Opportunistic Infections in a Rhesus Monkey Toxicology Study., PMID:35730205
Current concepts regarding Graves' orbitopathy., PMID:35604323
Management of Graves' hyperthyroidism: present and future., PMID:35287535
Quantitative evaluation of drug efficacy in the treatment of myasthenia gravis., PMID:34821184
Targeted Therapy for Primary Sjögren's Syndrome: Where are We Now?, PMID:34731460
Precision Medicine in Graves' Disease: CD40 Gene Variants Predict Clinical Response to an Anti-CD40 Monoclonal Antibody., PMID:34149627
[Sjögren's syndrome: from diagnosis to treatment]., PMID:33689243
Novel Approaches for Immunosuppression in Graves' Hyperthyroidism and Associated Orbitopathy., PMID:33511082
Developments in immunosuppression., PMID:33332922
Blocking T cell co-stimulation in primary Sjögren's syndrome: rationale, clinical efficacy and modulation of peripheral and salivary gland biomarkers., PMID:33095146
Biologics in the treatment of Sjogren's syndrome, systemic lupus erythematosus, and lupus nephritis., PMID:33002950
Assessment of the anti-CD40 antibody iscalimab in patients with primary Sjögren's syndrome: a multicentre, randomised, double-blind, placebo-controlled, proof-of-concept study., PMID:38263652
Sjögren's syndrome: Old and new therapeutic targets., PMID:31831255
First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody., PMID:31647605
A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial., PMID:31512728
Nonclinical Safety Assessment of CFZ533, a Fc-Silent Anti-CD40 Antibody, in Cynomolgus Monkeys., PMID:30099540
Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody., PMID:29665205
Current status of costimulatory blockade in renal transplantation., PMID:27517137