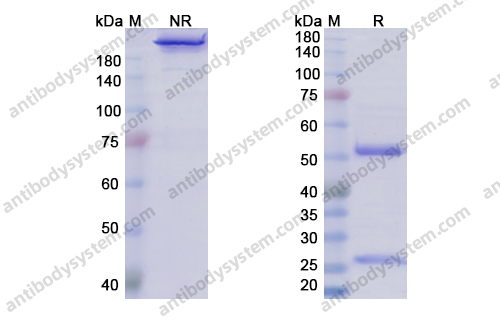
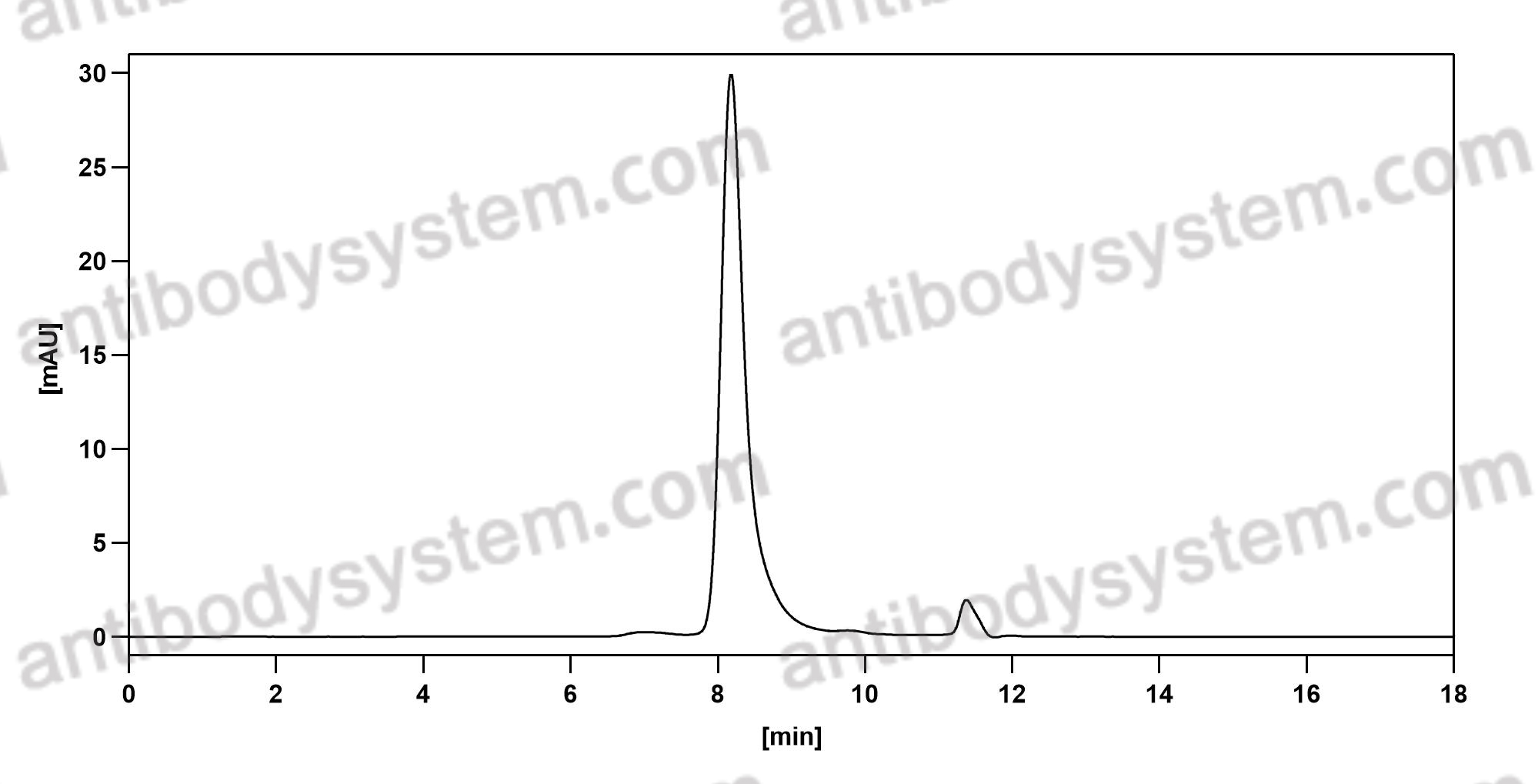
Catalog No.
DHD72201
Description
Varlilumab is a fully human monoclonal antibody (mAb) that targets CD27, a critical molecule in the activation pathway of lymphocytes. Like CD40, CD27 can be effectively manipulated with activating antibodies to induce potent anti-tumor responses, and may result in less toxicities due to its restricted expression and regulation. Varlilumab is a novel, first-in-class, agonist CD27 antibody that stimulates the CD27 pathway, which results in T-cell activation and antitumor activity in tumor models. This first-in-human, dose-escalation and expansion study evaluated the safety, pharmacology, and activity of varlilumab in patients with advanced solid tumors.
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
CD27L receptor, TNFRSF7, T14, CD27 antigen, Tumor necrosis factor receptor superfamily member 7, CD27, T-cell activation antigen CD27
Concentration
2 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P26842
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
1F5, CDX-1127, CAS: 1393344-72-3
Clone ID
Varlilumab
New emerging targets in cancer immunotherapy: CD27 (TNFRSF7), PMID: 32152062
Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies, PMID: 32380537
Safety and Activity of Varlilumab, a Novel and First-in-Class Agonist Anti-CD27 Antibody, in Patients With Advanced Solid Tumors, PMID: 28463630
Characterization of the human T cell response to in vitro CD27 costimulation with varlilumab, PMID: 26500773
RIVA - a phase IIa study of rituximab and varlilumab in relapsed or refractory B-cell malignancies: study protocol for a randomized controlled trial, PMID: 30413184
Immunothérapie des glioblastomes, PMID: 30595200
Conditioning treatment with CD27 Ab enhances expansion and antitumor activity of adoptively transferred T cells in mice, PMID: 34028568
CD27-Mediated Regulatory T Cell Depletion and Effector T Cell Costimulation Both Contribute to Antitumor Efficacy, PMID: 29109120
Antibody Tumor Targeting Is Enhanced by CD27 Agonists through Myeloid Recruitment, PMID: 29198913
PD-1 Blockade and CD27 Stimulation Activate Distinct Transcriptional Programs That Synergize for CD8 + T-Cell-Driven Antitumor Immunity, PMID: 29514845
Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia, PMID: 22589397
A Phase I Trial of Atezolizumab and Varlilumab in Combination With Radiation in Patients With Metastatic NSCLC., PMID:39161962
A combinatory vaccine with IMA950 plus varlilumab promotes effector memory T-cell differentiation in the peripheral blood of patients with low-grade gliomas., PMID:37758193
Production and N-glycan engineering of Varlilumab in Nicotiana benthamiana., PMID:37615027
FcγR requirements and costimulatory capacity of Urelumab, Utomilumab, and Varlilumab., PMID:37575254
Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors., PMID:35940825
Upregulation of the ErbB family by EZH2 in hepatocellular carcinoma confers resistance to FGFR inhibitor., PMID:34156519
Conditioning treatment with CD27 Ab enhances expansion and antitumor activity of adoptively transferred T cells in mice., PMID:34028568
Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies., PMID:32380537
New emerging targets in cancer immunotherapy: CD27 (TNFRSF7)., PMID:32152062
[Not Available]., PMID:30595200
RIVA - a phase IIa study of rituximab and varlilumab in relapsed or refractory B-cell malignancies: study protocol for a randomized controlled trial., PMID:30413184
PD-1 Blockade and CD27 Stimulation Activate Distinct Transcriptional Programs That Synergize for CD8+ T-Cell-Driven Antitumor Immunity., PMID:29514845
Antibody Tumor Targeting Is Enhanced by CD27 Agonists through Myeloid Recruitment., PMID:29198913
CD27-Mediated Regulatory T Cell Depletion and Effector T Cell Costimulation Both Contribute to Antitumor Efficacy., PMID:29109120
Safety and Activity of Varlilumab, a Novel and First-in-Class Agonist Anti-CD27 Antibody, in Patients With Advanced Solid Tumors., PMID:28463630
Characterization of the human T cell response to in vitro CD27 costimulation with varlilumab., PMID:26500773
Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia., PMID:22589397