Catalog No.
DHD62614
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
IL-4RA, IL-4-binding protein, IL-4 receptor subunit alpha, Soluble IL-4R-alpha, IL4-BP, IL4R, IL-4R subunit alpha, IL-4R-alpha, CD124, Interleukin-4 receptor subunit alpha, IL4RA, Soluble IL-4 receptor subunit alpha, sIL4Ralpha/prot
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
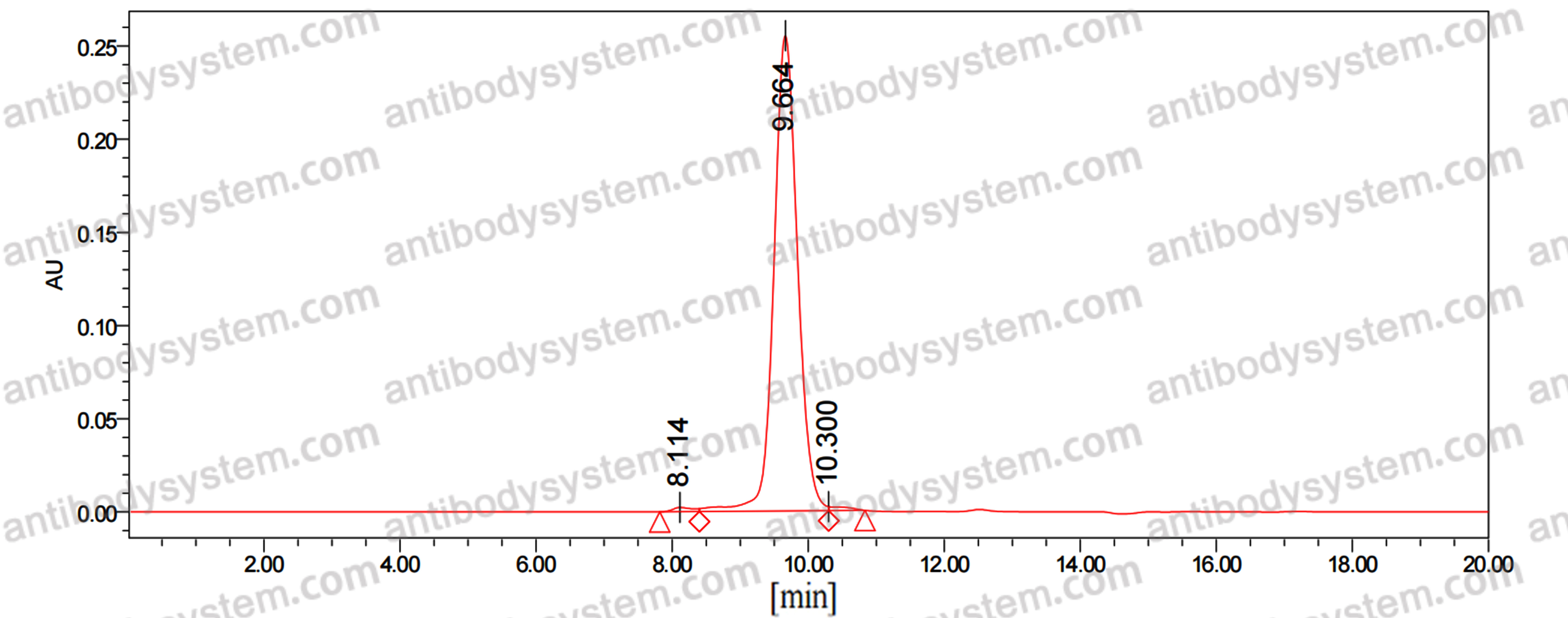
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P24394
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CAS: 2734715-10-5
Clone ID
Stapokibart
Stapokibart Rapidly and Consistently Improves the Clinical Signs Across All Body Regions in Adults With Moderate-to-Severe Atopic Dermatitis., PMID:40317961
Stapokibart for moderate-to-severe seasonal allergic rhinitis: a randomized phase 3 trial., PMID:40186079
Long-Term Stapokibart Treatment Induces Sustained Clinical Improvements in Patients With Moderate-to-Severe Atopic Dermatitis., PMID:40152029
Stapokibart: First Approval., PMID:40095376
Stapokibart (CM310) in patients with uncontrolled seasonal allergic rhinitis (PHECDA): Rationale and design of a multicentre, randomized, double-blind, placebo-controlled study., PMID:40051426
Long-term efficacy and safety of stapokibart for moderate-to-severe atopic dermatitis: 52-week results from a phase 3 trial., PMID:39450683
Stapokibart (CM310) targets IL-4Rα for the treatment of type 2 inflammation., PMID:39262798
Long-Term Efficacy and Safety of Stapokibart in Adults with Moderate-to-Severe Atopic Dermatitis: An Open-Label Extension, Nonrandomized Clinical Trial., PMID:39080181
Efficacy and safety of stapokibart (CM310) in adults with moderate-to-severe atopic dermatitis: A multicenter, randomized, double-blind, placebo-controlled phase 3 trial., PMID:39038559
Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Stapokibart in Healthy Volunteers and Adult Subjects with Atopic Dermatitis., PMID:38833140
Efficacy and safety of stapokibart (CM310) in uncontrolled seasonal allergic rhinitis (MERAK): an investigator-initiated, placebo-controlled, randomised, double-blind, phase 2 trial., PMID:38356731