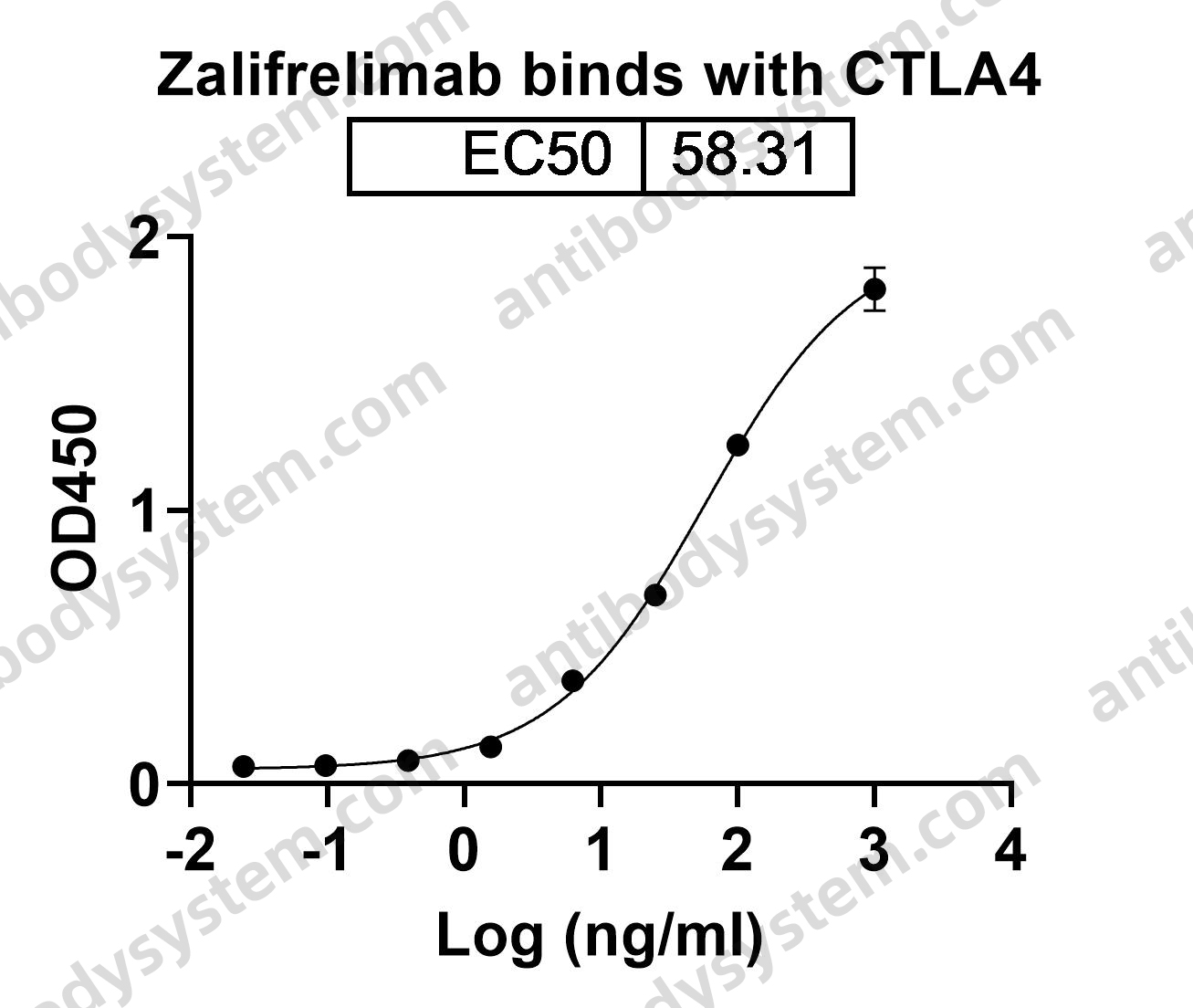
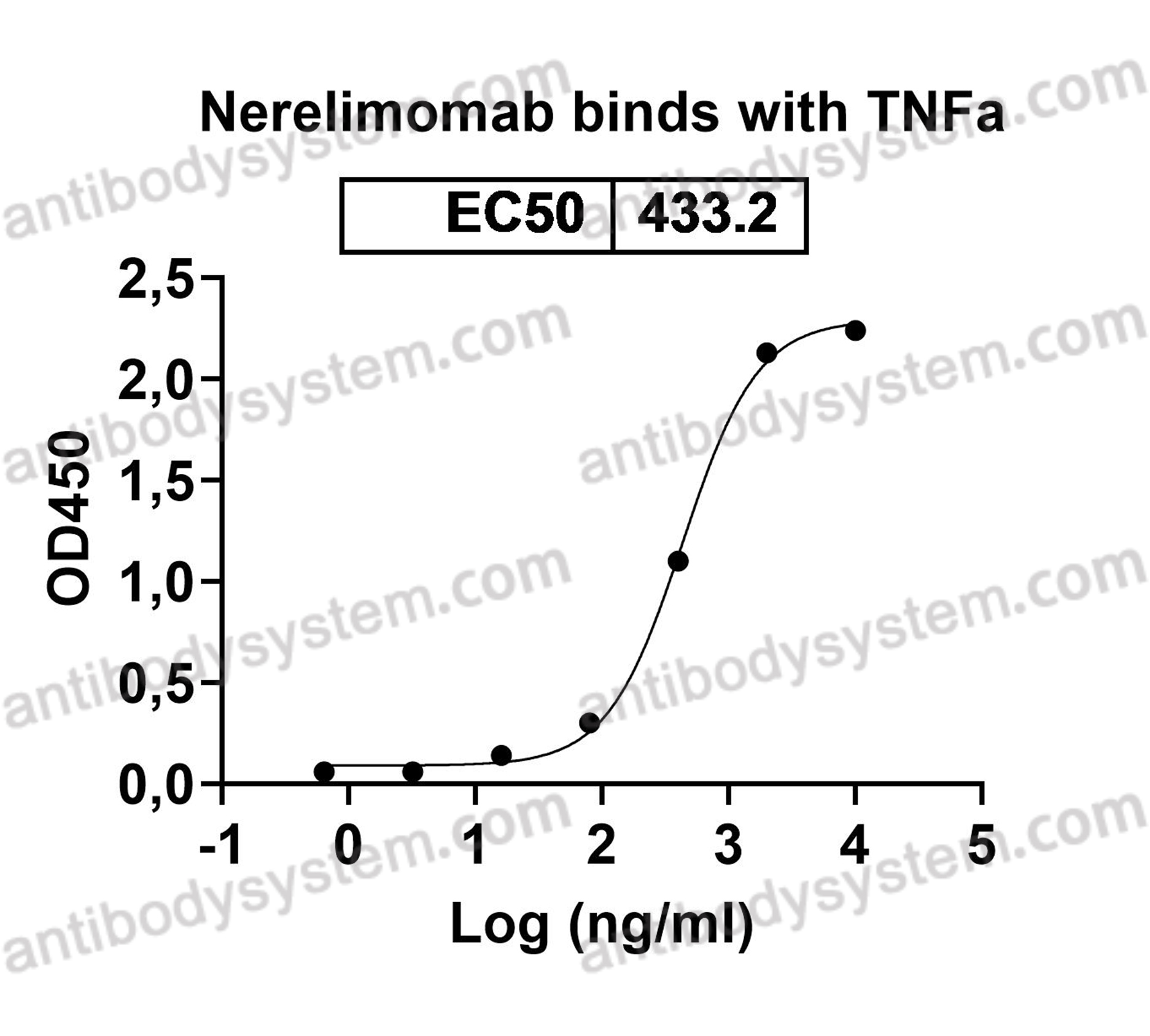
Anti-CD23, PMID: 16222084
Novel Drugs in Follicular Lymphoma, PMID: 27872741
Gateways to clinical trials, PMID: 12949633
State-of-the-art treatment of chronic lymphocytic leukemia, PMID: 20008230
Targeted therapy for chronic lymphocytic leukemia, PMID: 19343298
Refractory chronic lymphocytic leukemia--new therapeutic strategies, PMID: 21317446
Antibody therapy for chronic lymphocytic leukemia, PMID: 18381104
Monoclonal antibodies in chronic lymphocytic leukemia, PMID: 17020457
Newer monoclonal antibodies for hematological malignancies, PMID: 18565392
Current and emerging monoclonal antibody treatments for chronic lymphocytic leukemia: state of the art, PMID: 25249370
Monoclonal antibody therapy of chronic lymphocytic leukemia, PMID: 16187090
Treatment of relapsed or refractory chronic lymphocytic leukemia, PMID: 22143061
Novel antibodies in the treatment of non-Hodgkin's lymphoma, PMID: 19767657
Modern concepts in the treatment of chronic lymphocytic leukemia, PMID: 19843378
Standard of care and novel treatments for chronic lymphocytic leukemia, PMID: 20966144
Novel monoclonal antibodies for the treatment of chronic lymphocytic leukemia, PMID: 18336199
Gateways to clinical trials, PMID: 21225019
Gateways to clinical trials, PMID: 18985183
Novel agents for the treatment of chronic lymphocytic leukemia, PMID: 21326166
Technology evaluation: lumiliximab, Biogen Idec, PMID: 15663332
Current and emerging treatments for chronic lymphocytic leukaemia, PMID: 19911856
The use of monoclonal antibodies in the treatment of autoimmune complications of chronic lymphocytic leukemia, PMID: 23667725
Variable contribution of monoclonal antibodies to ADCC in patients with chronic lymphocytic leukemia, PMID: 19562616
Anti-CD23 monoclonal antibody, lumiliximab, inhibited allergen-induced responses in antigen-presenting cells and T cells from atopic subjects, PMID: 16210051
Mediation of apoptosis by and antitumor activity of lumiliximab in chronic lymphocytic leukemia cells and CD23+ lymphoma cell lines, PMID: 18032710
A randomized, open-label, multicentre, phase 2/3 study to evaluate the safety and efficacy of lumiliximab in combination with fludarabine, cyclophosphamide and rituximab versus fludarabine, cyclophosphamide and rituximab alone in subjects with relapsed chronic lymphocytic leukaemia, PMID: 25130401
Phase behavior of an intact monoclonal antibody, PMID: 17449660
Phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia, PMID: 19843887
Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia, PMID: 17671129
Isolation of human Fab antibodies specific for the low-affinity IgE receptor (CD23) by selecting a hierarchical antibody library system against B lymphoblastic IM-9 cells, PMID: 21277901
IgE directly affects eosinophil migration in chronic rhinosinusitis with nasal polyps through CCR3 and predicts the efficacy of omalizumab., PMID:37922997
Novel Drugs in Follicular Lymphoma., PMID:27872741
Current and emerging monoclonal antibody treatments for chronic lymphocytic leukemia: state of the art., PMID:25249370
A randomized, open-label, multicentre, phase 2/3 study to evaluate the safety and efficacy of lumiliximab in combination with fludarabine, cyclophosphamide and rituximab versus fludarabine, cyclophosphamide and rituximab alone in subjects with relapsed chronic lymphocytic leukaemia., PMID:25130401
The use of monoclonal antibodies in the treatment of autoimmune complications of chronic lymphocytic leukemia., PMID:23667725
Treatment of relapsed or refractory chronic lymphocytic leukemia., PMID:22143061
Novel agents for the treatment of chronic lymphocytic leukemia., PMID:21326166
Refractory chronic lymphocytic leukemia--new therapeutic strategies., PMID:21317446
Isolation of human Fab antibodies specific for the low-affinity IgE receptor (CD23) by selecting a hierarchical antibody library system against B lymphoblastic IM-9 cells., PMID:21277901
Gateways to clinical trials., PMID:21225019
Standard of care and novel treatments for chronic lymphocytic leukemia., PMID:20966144
State-of-the-art treatment of chronic lymphocytic leukemia., PMID:20008230
Current and emerging treatments for chronic lymphocytic leukaemia., PMID:19911856
Phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia., PMID:19843887
Modern concepts in the treatment of chronic lymphocytic leukemia., PMID:19843378
Novel antibodies in the treatment of non-Hodgkin's lymphoma., PMID:19767657
Variable contribution of monoclonal antibodies to ADCC in patients with chronic lymphocytic leukemia., PMID:19562616
Targeted therapy for chronic lymphocytic leukemia., PMID:19343298
Gateways to clinical trials., PMID:18985183
Newer monoclonal antibodies for hematological malignancies., PMID:18565392
Antibody therapy for chronic lymphocytic leukemia., PMID:18381104
Novel monoclonal antibodies for the treatment of chronic lymphocytic leukemia., PMID:18336199
Mediation of apoptosis by and antitumor activity of lumiliximab in chronic lymphocytic leukemia cells and CD23+ lymphoma cell lines., PMID:18032710
Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia., PMID:17671129
Phase behavior of an intact monoclonal antibody., PMID:17449660
Monoclonal antibodies in chronic lymphocytic leukemia., PMID:17020457
Anti-CD23., PMID:16222084
Anti-CD23 monoclonal antibody, lumiliximab, inhibited allergen-induced responses in antigen-presenting cells and T cells from atopic subjects., PMID:16210051
Monoclonal antibody therapy of chronic lymphocytic leukemia., PMID:16187090
Technology evaluation: lumiliximab, Biogen Idec., PMID:15663332
Gateways to clinical trials., PMID:12949633