Catalog No.
PHC82801
Species reactivity
Human
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant Human TFPI (Asp29-Phe209).
Tested applications
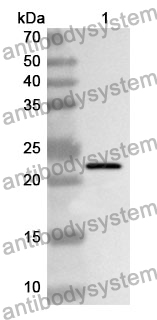
ELISA: 1:4000-1:8000, IHC: 1:50-1:100, WB: 1:1000-1:4000
Target
Extrinsic pathway inhibitor,Lipoprotein-associated coagulation inhibitor,TFPI,EPI,LACI,TFPI1,Tissue factor pathway inhibitor
Purification
Purified by antigen affinity column.
Accession
P10646
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Safety and efficacy of marstacimab in patients with hemophilia A and B: a systematic review and meta-analysis., PMID:40528319
Evaluating the Safety and Efficacy of Concizumab in Hemophilia A/B Patients: A Systematic Review., PMID:40368339
Revolutionizing Treatment Strategies through Inhibition of Tissue Factor Pathway Inhibitor: A Promising Therapeutic Approach for Hemophilia Management., PMID:40200623
Novel drugs approved by the EMA, the FDA and the MHRA in 2024: A year in review., PMID:39971274
Marstacimab: First Approval., PMID:39715914
Examining downstream effects of concizumab in hemophilia A with a mathematical modeling approach., PMID:39536817
Functionally distinct anticoagulant mechanisms of endothelial cells., PMID:39522336
FDA approves first anti-TFPI antibody for haemophilia A and B., PMID:39478181
[Advances in hemophilia treatment]., PMID:39358264
Deletion of tissue factor pathway inhibitor isoform beta or gamma, but not alpha, improves clotting in hemophilic mice., PMID:38925489
Minimal interference of concizumab with standard clinical coagulation laboratory assays - An in vitro study., PMID:38924198
Targeting tissue factor pathway inhibitor with concizumab to improve hemostasis in patients with Glanzmann thrombasthenia: an in vitro study., PMID:38880178
Concizumab improves clot formation in hemophilia A under flow., PMID:38815755
Impact of tissue factor expression and administration routes on thrombosis development induced by mesenchymal stem/stromal cell infusions: re-evaluating the dogma., PMID:38414067
Unfolded Von Willebrand Factor Binds Protein S and Reduces Anticoagulant Activity., PMID:38370737
TFPIα anticoagulant function is highly dependent on protein S in vivo., PMID:38306422
Thrombin generation on vascular cells in the presence of factor VIII and/or emicizumab., PMID:37776978
Activated protein C, protein S, and tissue factor pathway inhibitor cooperate to inhibit thrombin activation., PMID:37660436
Evolution of Antidrug Antibody Assays During the Development of Anti-Tissue Factor Pathway Inhibitor Monoclonal Antibody Marstacimab., PMID:37610502
Concizumab: First Approval., PMID:37341887
Tissue factor pathway inhibitor is a potential modifier of bleeding risk in factor XI deficiency., PMID:36696199
Absence of Effect of Emicizumab on D-Dimer Concentrations in Adult Patients with Severe Hemophilia A., PMID:36474347
SARS-CoV-2 vaccines are not associated with hypercoagulability in apparently healthy people., PMID:36448024
A phase 1b/2 clinical study of marstacimab, targeting human tissue factor pathway inhibitor, in haemophilia., PMID:35999026
Human IL-17 and TNF-α Additively or Synergistically Regulate the Expression of Proinflammatory Genes, Coagulation-Related Genes, and Tight Junction Genes in Porcine Aortic Endothelial Cells., PMID:35844613
Profiling of extracellular vesicles of metastatic urothelial cancer patients to discover protein signatures related to treatment outcome., PMID:35838333
Befovacimab, an anti-tissue factor pathway inhibitor antibody: Early termination of the multiple-dose, dose-escalating Phase 2 study due to thrombosis., PMID:35667016
Concizumab as a Subcutaneous Prophylactic Treatment Option for Patients with Hemophilia A or B: A Review of the Evidence and Patient's Perspectives., PMID:35465188
Revised model of the tissue factor pathway of thrombin generation: Role of the feedback activation of FXI., PMID:35352494
Hemostatic efficacy of marstacimab alone or in combination with bypassing agents in hemophilia plasmas and a mouse bleeding model., PMID:35316941
Regulation of coagulation by tissue factor pathway inhibitor: Implications for hemophilia therapy., PMID:35279938
Non-severe COVID-19 is associated with endothelial damage and hypercoagulability despite pharmacological thromboprophylaxis., PMID:35102689
Clinical Utility of Subcutaneous Factor VIII Replacement Therapies in Hemophilia A: A Review of the Evidence., PMID:34908888
Factor V east Texas variant causes bleeding in a three-generation family., PMID:34847292
Resistance to activated protein C and impaired TFPI activity in women with previous hormone-induced venous thromboembolism., PMID:34634502
Evaluation of the efficacy of a novel Vibrio vulnificus vaccine based on antibacterial peptide inactivation in turbot, Scophthalmus maximus., PMID:34509628
Pulmonary coagulation and fibrinolysis abnormalities that favor fibrin deposition in the lungs of mouse antibody-mediated transfusion-related acute lung injury., PMID:34165170
Progress in the Development of Anti-tissue Factor Pathway Inhibitors for Haemophilia Management., PMID:34026796
An in vitro pharmacodynamic spiking study of befovacimab, a tissue factor pathway inhibitor monoclonal antibody, in blood samples from patients with severe FVIII deficiency., PMID:33915599
Use of population PK/PD approach to model the thrombin generation assay: assessment in haemophilia A plasma samples spiked by a TFPI antibody., PMID:33846873
Tissue factor pathway inhibitor upregulates CXCR7 expression and enhances CXCL12-mediated migration in chronic lymphocytic leukemia., PMID:33664415
Concizumab: a novel anti-TFPI therapeutic for hemophilia., PMID:33570646
Target-mediated drug disposition modeling of an anti-TFPI antibody (MG1113) in cynomolgus monkeys to predict human pharmacokinetics and pharmacodynamics., PMID:33448093
MG1113, a specific anti-tissue factor pathway inhibitor antibody, rebalances the coagulation system and promotes hemostasis in hemophilia., PMID:33313469
Yersinia pestis Plasminogen Activator., PMID:33202679
[Analysis of Transfusion Therapy Effectiveness in Patients with Myelodysplastic Syndrome]., PMID:33067968
A new hybrid immunocapture bioassay with improved reproducibility to measure tissue factor-dependent procoagulant activity of microvesicles from body fluids., PMID:33038585
Proteomic identification of Placental Protein 1 (PP1), PP8, and PP22 and characterization of their placental expression in healthy pregnancies and in preeclampsia., PMID:32747003
The Evolution of Hemophilia Care: Clinical and Laboratory Advances, Opportunities, and Challenges., PMID:32726826