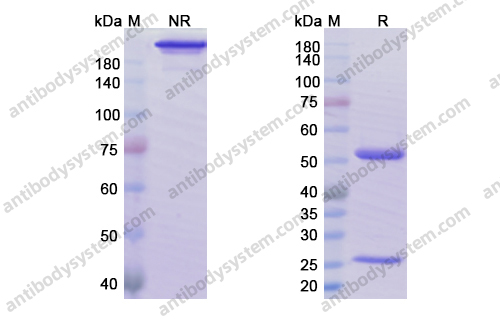
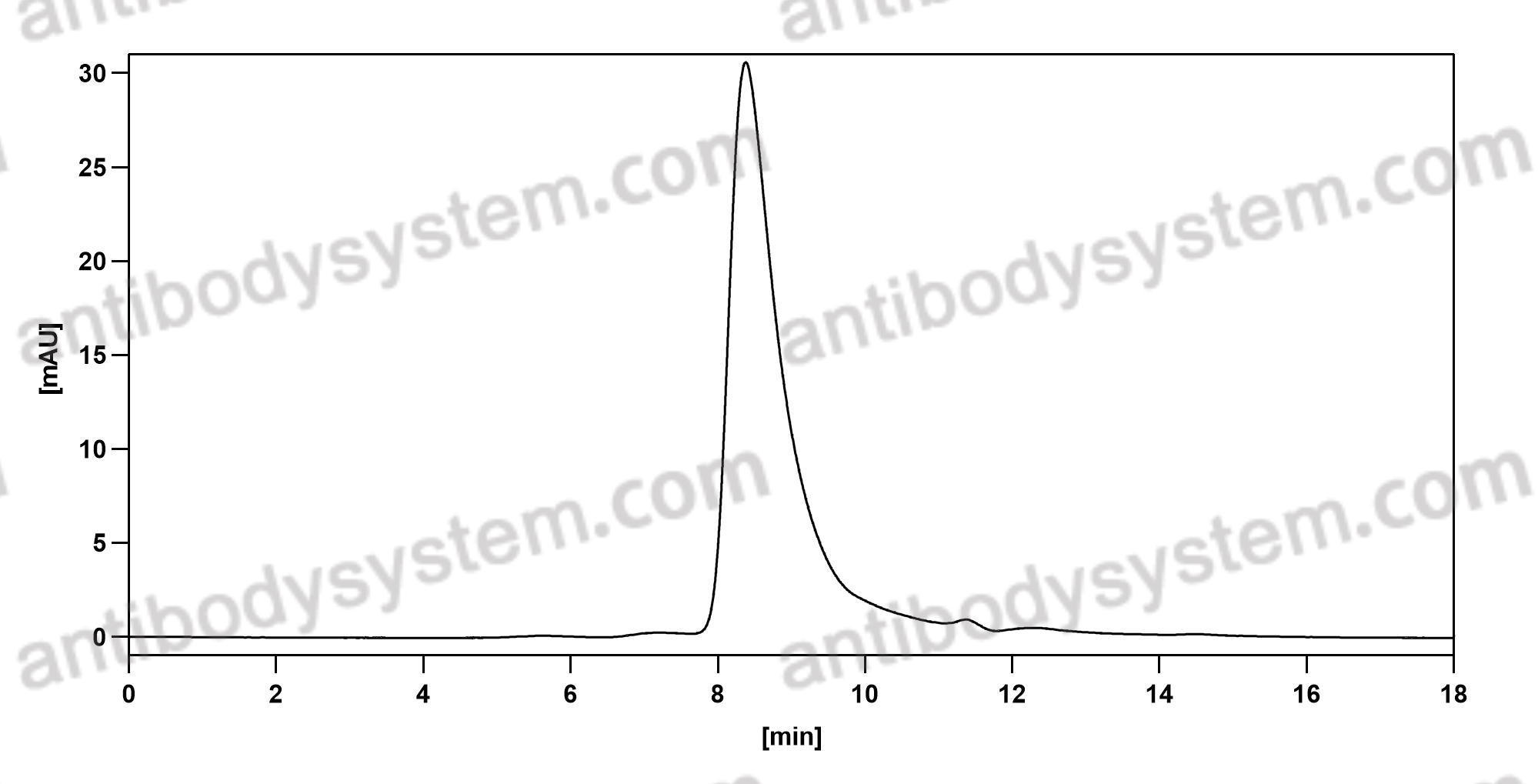
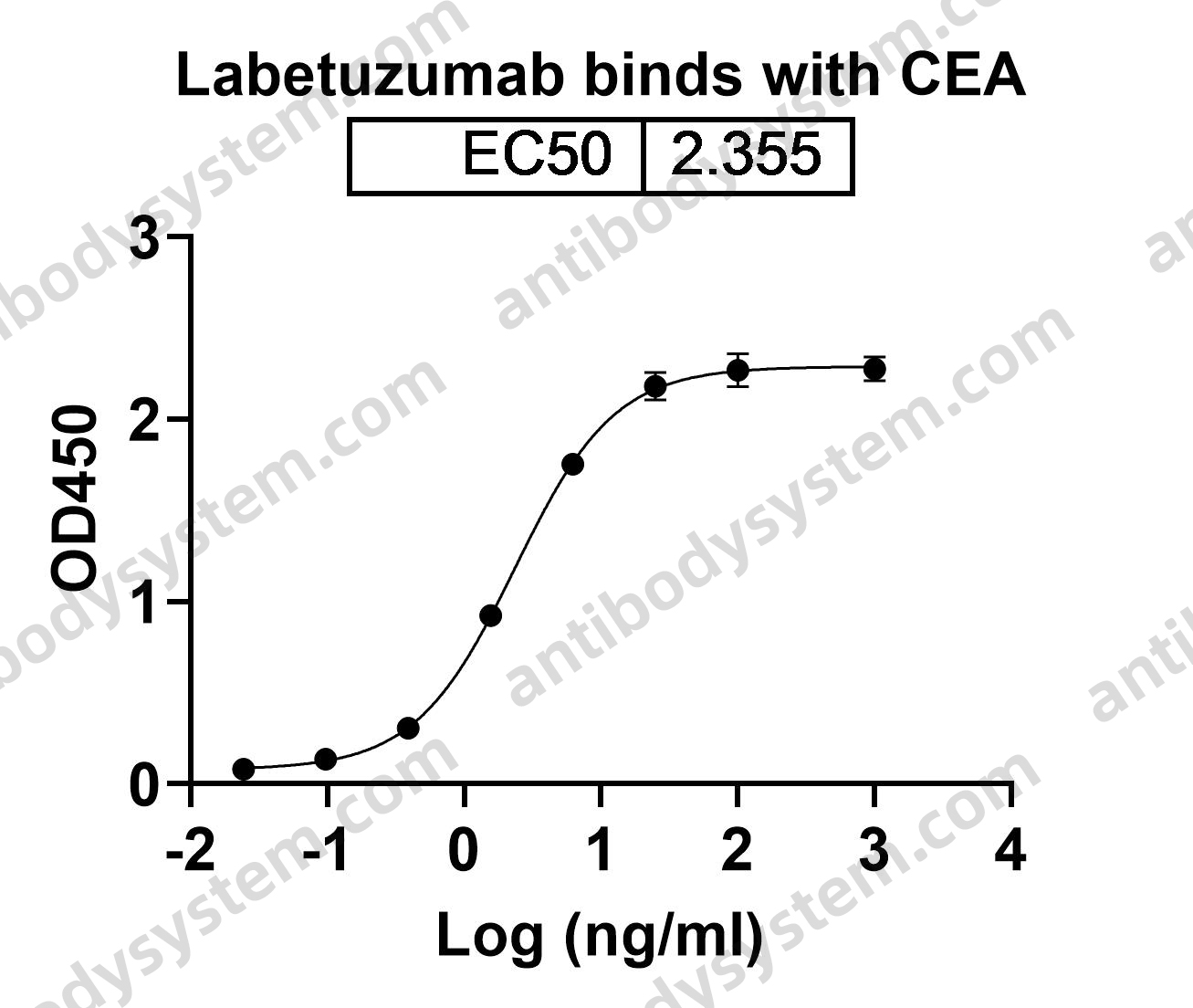
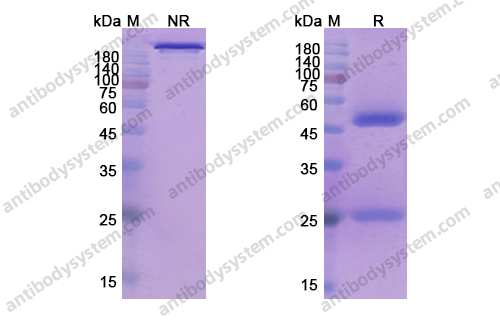
Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, PMID: 31558467
A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis, PMID: 32351164
Ublituximab for the treatment of CD20 positive B-cell malignancies, PMID: 29609506
Antibodies to watch in 2020, PMID: 31847708
Antibodies to watch in 2021, PMID: 33459118
Antibodies to watch in 2019, PMID: 30516432
Immunotherapy in myasthenia gravis in the era of biologics, PMID: 30573759
Therapies for multiple sclerosis targeting B cells, PMID: 31044580
Targeting B cells to modify MS, NMOSD, and MOGAD: Part 2, PMID: 33411674
Anti-CD20 Monoclonal Antibodies for Relapsing and Progressive Multiple Sclerosis, PMID: 31994023
CD20 monoclonal antibodies for the treatment of multiple sclerosis: up-to-date, PMID: 31027436
Targeting B Cells to Modify MS, NMOSD, and MOGAD: Part 1, PMID: 33406479
B cell depletion with ublituximab reshapes the T cell profile in multiple sclerosis patients, PMID: 31077854
Anti-CD20 treatment for B-cell malignancies: current status and future directions, PMID: 32933335
A pilot safety study of ublituximab, a monoclonal antibody against CD20, in acute relapses of neuromyelitis optica spectrum disorder, PMID: 31232925
Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial, PMID: 27982425
Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial, PMID: 30709431
Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): a phase 3, multicentre, open-label, randomised trial, PMID: 33631112
Preclinical study of Ublituximab, a Glycoengineered anti-human CD20 antibody, in murine models of primary cerebral and intraocular B-cell lymphomas, PMID: 23611989
A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab, PMID: 28220479
The optimized anti-CD20 monoclonal antibody ublituximab bypasses natural killer phenotypic features in Waldenström macroglobulinemia, PMID: 25552707
Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion, PMID: 23958919
Possible novel agents in marginal zone lymphoma, PMID: 28288710
Antibodies to watch in 2018, PMID: 29300693
Anti-CD20 B Cell Treatment for Relapsing Multiple Sclerosis, PMID: 33551958
Dancing partners at the ball: Rational selection of next generation anti-CD20 antibodies for combination therapy of chronic lymphocytic leukemia in the novel agents era, PMID: 28499646
Efficacy of Ibrutinib-Based Regimen in Chronic Lymphocytic Leukemia: A Systematic Review, PMID: 32300434
Humoral immune response in convalescent COVID-19 people with multiple sclerosis treated with high-efficacy disease-modifying therapies: A multicenter, case-control study, PMID: 34418815
B cell depletion changes the immune cell profile in multiple sclerosis patients: One-year report, PMID: 34364105
Antibodies to watch in 2017, PMID: 27960628
Anti-CD20 therapies for multiple sclerosis: current status and future perspectives, PMID: 34382120
B Cells and Antibodies as Targets of Therapeutic Intervention in Neuromyelitis Optica Spectrum Disorders, PMID: 33419217
Comparison of the Efficacy and Safety of Anti-CD20 B Cells Depleting Drugs in Multiple Sclerosis, PMID: 33516134
Ibrutinib continues to influence the therapeutic landscape of chronic lymphocytic leukemia: new data presented at ASCO 2017, PMID: 28810856
Clinical Perspectives on the Molecular and Pharmacological Attributes of Anti-CD20 Therapies for Multiple Sclerosis, PMID: 34370283
Anti-CD20 monoclonal antibody-dependent phagocytosis of chronic lymphocytic leukaemia cells by autologous macrophages, PMID: 26307241
A Milestone in Multiple Sclerosis Therapy: Monoclonal Antibodies Against CD20-Yet Progress Continues, PMID: 33880738
Switching to ublituximab from prior anti-CD20 monoclonal antibody therapy: a case report series., PMID:40255393
The Evolution of Anti-CD20 Treatment for Multiple Sclerosis: Optimization of Antibody Characteristics and Function., PMID:40180777
A chemotherapy-free regimen of acalabrutinib, umbralisib and ublituximab achieved high response rates and undetectable minimal residual disease in patients with untreated mantle cell lymphoma., PMID:40176303
Treatment of Relapsed/Refractory CLL Patients With PI3Kδ Inhibitor and Anti-CD20 Antibody Rapidly Decreases Tumor Burden but Could Induce Resistance., PMID:39745891
Exploring Public Interest in Multiple Sclerosis and Its Treatment Measures in the United States: A Google Trends Analysis., PMID:39734957
Comparative efficacy and tolerability of ublituximab vs. other monoclonal antibodies in the treatment of relapsing multiple sclerosis: a systematic review and network meta-analysis of randomized trials., PMID:39711787
Recurrent late-onset neutropenia following treatment with different B cell-depleting strategies in multiple sclerosis., PMID:39515320
Improvements in no evidence of disease activity with ublituximab vs. teriflunomide in the ULTIMATE phase 3 studies in relapsing multiple sclerosis., PMID:39512280
B-cell Depletion Therapy in Pediatric Neuroinflammatory Disease., PMID:39259430
Evaluating the Therapeutic Potential of Ublituximab in the Treatment of MS: Design, Development and Place in Therapy., PMID:39050801
Ocrelizumab-induced organizing pneumonia in multiple sclerosis: case report and literature review., PMID:39015300
Safety profile of first-line targeted therapies in elderly and/or comorbid chronic lymphocytic leukaemia patients (unfit subpopulation). A systematic review and network meta-analysis., PMID:38969250
Drugs Targeting CD20 in Multiple Sclerosis: Pharmacology, Efficacy, Safety, and Tolerability., PMID:38480630
The course of COVID-19 in a multiple sclerosis: a case report., PMID:38431822
A Proposed Approach to Screening and Surveillance Labs for Patients With Multiple Sclerosis on Anti-CD20 Therapy., PMID:38204588
Neutropenia following immune-depletion, notably CD20 targeting, therapies in multiple sclerosis., PMID:38181696
Key characteristics of anti-CD20 monoclonal antibodies and clinical implications for multiple sclerosis treatment., PMID:37906325
Response-adapted, time-limited venetoclax, umbralisib, and ublituximab for relapsed/refractory chronic lymphocytic leukemia., PMID:37871300
Ublituximab-xiiy as a treatment option for relapsing multiple sclerosis., PMID:37842819
Briumvi: a breakthrough in the treatment of relapsing multiple sclerosis: a review., PMID:37811115
Secondary hypogammaglobulinemia in patients with multiple sclerosis on anti-CD20 therapy: Pathogenesis, risk of infection, and disease management., PMID:37783194
Health-economic benefits of anti-CD20 treatments in relapsing multiple sclerosis estimated using a treatment-sequence model., PMID:37529628
Phase 2 trial of umbralisib, ublituximab, and venetoclax in patients with relapsed/refractory mantle cell lymphoma., PMID:37341984
Comparative efficacy of therapies for relapsing multiple sclerosis: a systematic review and network meta-analysis., PMID:37265062
Monoclonal Antibodies in Pregnancy and Breastfeeding in Patients with Multiple Sclerosis: A Review and an Updated Clinical Guide., PMID:37242553
Ublituximab: A new FDA-approved anti-CD20 mAb for relapsing forms of multiple sclerosis., PMID:37156035
G protein-coupled receptor 183 mediates the sensitization of Burkitt lymphoma tumors to CD47 immune checkpoint blockade by anti-CD20/PI3Kδi dual therapy., PMID:37153563
Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic., PMID:37033984
Targeting CD20 in multiple sclerosis - review of current treatment strategies., PMID:36999373
Ublituximab: First Approval., PMID:36920653
Ublituximab (Briumvi) for relapsing multiple sclerosis., PMID:36877282
Ublituximab-xiiy., PMID:36846893
Ublituximab: a new anti-CD20 agent for multiple sclerosis., PMID:36402153
The ULTIMATE trials: are there advantages of ublituximab over teriflunomide in relapsing multiple sclerosis?, PMID:36317532
Progressive multifocal leukoencephalopathy in anti-CD20 and other monoclonal antibody (mAb) therapies used in multiple sclerosis: A review., PMID:36283150
B-Cell Targeted Therapies in Patients with Multiple Sclerosis and Incidence of Headache: A Systematic Review and Meta-Analysis., PMID:36143259
Updates on efficacy and safety outcomes of new and emerging disease modifying therapies and stem cell therapy for Multiple Sclerosis: A review., PMID:36057173
Efficacy and safety of ibrutinib in mantle cell lymphoma: A systematic review and meta-analysis., PMID:36057010
Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis., PMID:36001711
Efficacy and Safety of Multiple Sclerosis Drugs Approved Since 2018 and Future Developments., PMID:35869335
Adding Umbralisib and Ublituximab (U2) to Ibrutinib in Patients with CLL: A Phase II Study of an MRD-Driven Approach., PMID:35852793
Outcomes of Ublituximab compared to Teriflunomide for relapsing multiple sclerosis: A meta-analysis., PMID:35779372
B-Cell-Directed Therapies: A New Era in Multiple Sclerosis Treatment., PMID:35570581
Monoclonal Antibodies in the Treatment of Relapsing Multiple Sclerosis: an Overview with Emphasis on Pregnancy, Vaccination, and Risk Management., PMID:35378683
Emerging drugs for the acute treatment of relapses in adult neuromyelitis optica spectrum disorder patients., PMID:35341428
Mantle cell lymphoma management trends and novel agents: where are we going?, PMID:35237397