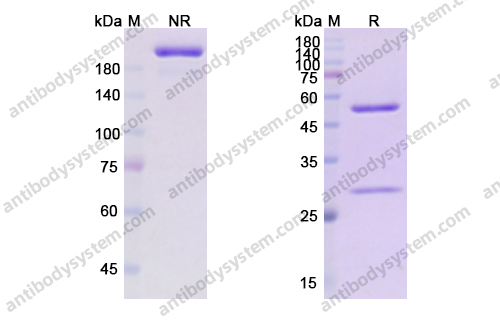
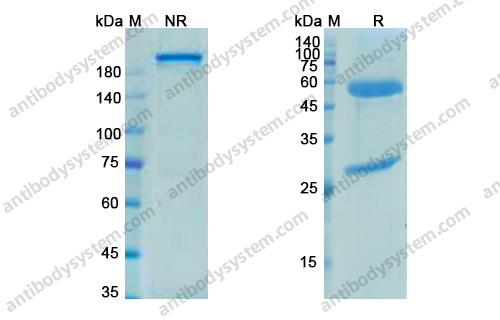
Catalog No.
DHD10807
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Chimeric
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
CD19, Differentiation antigen CD19, T-cell surface antigen Leu-12, B-lymphocyte antigen CD19, B-lymphocyte surface antigen B4
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P15391
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
XmAb5871, CAS: 1690307-05-1
Clone ID
Obexelimab
Emerging B-Cell Therapies in Systemic Lupus Erythematosus, PMID: 33488082
Bispecific Antibodies for Autoimmune and Inflammatory Diseases: Clinical Progress to Date, PMID: 31916225
Inhibition of B cell activation following in vivo co-engagement of B cell antigen receptor and Fcγ receptor IIb in non-autoimmune-prone and SLE-prone mice, PMID: 33409482
Pharmacokinetics, Receptor Occupancy, and Pharmacodynamics of Obexelimab Following Intravenous Administration in Adult Healthy Volunteers and in Patients With Rheumatoid Arthritis., PMID:40512061
IgG4-related disease: lessons from the first 20 years., PMID:40071397
Pharmacokinetics, Pharmacodynamics, Bioavailability, and Immunogenicity of Obexelimab Following Subcutaneous Administration in Healthy Japanese and Non-Japanese Volunteers., PMID:39636565
Autoimmune Hemolytic Anemias: Challenges in Diagnosis and Therapy., PMID:39371250
Evaluation of the safety, efficacy, and mechanism of action of obexelimab for the treatment of patients with IgG4-related disease: an open-label, single-arm, single centre, phase 2 pilot trial., PMID:38251576
Obexelimab in IgG4-related disease: B-cell inhibition as a novel therapeutic approach., PMID:38251568
Antibody based therapeutics for autoimmune hemolytic anemia., PMID:37874225
[IgG4-related disease: A proteiform pathology with frequent chest manifestations]., PMID:37858433
Advances in natural products and antibody drugs for SLE: new therapeutic ideas., PMID:37492083
Obexelimab in Systemic Lupus Erythematosus With Exploration of Response Based on Gene Pathway Co-Expression Patterns: A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial., PMID:37459248
Autoimmune Neurological Disorders with IgG4 Antibodies: a Distinct Disease Spectrum with Unique IgG4 Functions Responding to Anti-B Cell Therapies., PMID:35290608
Emerging B-Cell Therapies in Systemic Lupus Erythematosus., PMID:33488082
Inhibition of B cell activation following in vivo co-engagement of B cell antigen receptor and Fcγ receptor IIb in non-autoimmune-prone and SLE-prone mice., PMID:33409482
Bispecific Antibodies for Autoimmune and Inflammatory Diseases: Clinical Progress to Date., PMID:31916225