Catalog No.
DHK16101
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
SAF2, Sialoadhesin family member 2, SAF-2, Siglec-8, SIGLEC8, Sialic acid-binding Ig-like lectin 8
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
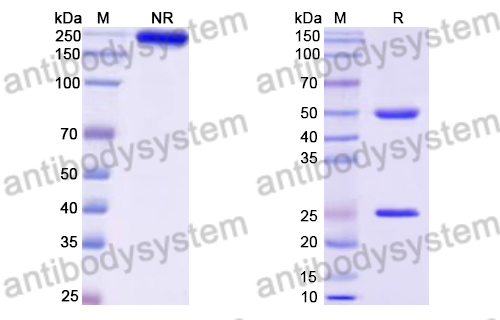
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9NYZ4
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
AK-002, AK002
Clone ID
Lirentelimab
New treatments for systemic mastocytosis in 2025., PMID:40471046
Hypereosinophilia: clinical and therapeutic approach in 2025., PMID:40396537
Pharmacologic Treatment of Eosinophilic Esophagitis: Efficacious, Likely Efficacious, and Failed Drugs., PMID:39474328
The New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis: Biological Drugs., PMID:38338983
Mast Cell-Targeting Therapies in Mast Cell Activation Syndromes., PMID:38217824
A study of the safety and effectiveness of lirentelimab as a treatment for people with indolent systemic mastocytosis., PMID:37879742
Non-esophageal eosinophilic gastrointestinal diseases: a narrative review., PMID:37814561
Mast cell silencing: A novel therapeutic approach for urticaria and other mast cell-mediated diseases., PMID:37605867
Comparison of drugs for active eosinophilic oesophagitis: systematic review and network meta-analysis., PMID:37491157
Is lirentelimab the 'magic bullet' to fight pathological mast-cell activation in systemic mastocytosis?, PMID:37403635
Safety and efficacy of lirentelimab in patients with refractory indolent systemic mastocytosis: a first-in-human clinical trial., PMID:37290787
Emerging therapies for eosinophilic esophagitis., PMID:36840634
[Allergology: what's new in 2022]., PMID:36723646
Structures of the Inhibitory Receptor Siglec-8 in Complex with a High-Affinity Sialoside Analogue and a Therapeutic Antibody., PMID:36711084
Current and emerging biologic therapies targeting eosinophilic disorders., PMID:35983569
Biologics in eosinophilic gastrointestinal diseases., PMID:35738437
Primary eosinophilic gastrointestinal disorders and allergy: Clinical and therapeutic implications., PMID:35620572
Update on Emerging Pharmacologic Therapies for Patients With Eosinophilic Esophagitis., PMID:35505944
Lirentelimab for severe and chronic forms of allergic conjunctivitis., PMID:35390403
An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria., PMID:34954198
Mast cell activation is associated with post-acute COVID-19 syndrome., PMID:34820848
Determination of Biopsy Yield That Optimally Detects Eosinophilic Gastritis and/or Duodenitis in a Randomized Trial of Lirentelimab., PMID:34089846
Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody., PMID:33777047
Biologics for the Use in Chronic Spontaneous Urticaria: When and Which., PMID:33685605
Targeting the FcεRI Pathway as a Potential Strategy to Prevent Food-Induced Anaphylaxis., PMID:33391286
Discovery, Function, and Therapeutic Targeting of Siglec-8., PMID:33374255
Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis., PMID:33085861