Catalog No.
DXX00201
Expression system
Mammalian Cells
Species reactivity
Clostridioides difficile
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Toxin A/tcdA
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
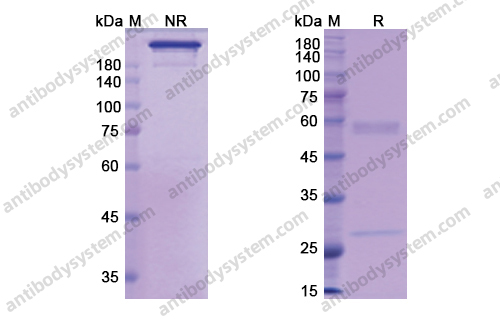
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P16154
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CDA 1, MBL-CDA1, MDX-066, MK-3415A (combination of actoxumab and bezlotoxumab), CAS: 1245634-25-6
Clone ID
Actoxumab
Monoclonal antibody-mediated neutralization of Clostridioides difficile toxin does not diminish induction of the protective innate immune response to infection., PMID:38701911
Biofilm Formation of Clostridioides difficile, Toxin Production and Alternatives to Conventional Antibiotics in the Treatment of CDI., PMID:37764005
Structure-guided design of a potent Clostridiodes difficile toxin A inhibitor., PMID:36778856
Add-on interventions for the prevention of recurrent Clostridioides Difficile infection: A systematic review and network meta-analysis., PMID:34454094
Immune response against Clostridioides difficile and translation to therapy., PMID:33995585
AAV-mediated delivery of actoxumab and bezlotoxumab results in serum and mucosal antibody concentrations that provide protection from C. difficile toxin challenge., PMID:33608675
Efficacy of Bezlotoxumab in Trial Participants Infected With Clostridioides difficile Strain BI Associated With Poor Outcomes., PMID:32735653
Efficacy and Safety of Monoclonal Antibodies Against Clostridioides difficile Toxins for Prevention of Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis., PMID:32053529
Bezlotoxumab for the Prevention of Recurrent Clostridioides difficile Infection: 12-Month Observational Data From the Randomized Phase III Trial, MODIFY II., PMID:31883370
Effect of Endogenous Clostridioides difficile Toxin Antibodies on Recurrence of C. difficile Infection., PMID:31628838
The Impact of Actotoxumab Treatment of Gnotobiotic Piglets Infected With Different Clostridium difficile Isogenic Mutants., PMID:31495879
Prevention of recurrent Clostridioides difficile infection: A systematic review of randomized controlled trials., PMID:31493500
Development of Neutralizing and Non-neutralizing Antibodies Targeting Known and Novel Epitopes of TcdB of Clostridioides difficile., PMID:30574127
Bezlotoxumab., PMID:30020417
Actoxumab + bezlotoxumab combination: what promise for Clostridium difficile treatment?, PMID:29534621
The effect of bezlotoxumab for prevention of recurrent Clostridium difficile infection (CDI) in Japanese patients., PMID:29097028
Bezlotoxumab for the treatment of Clostridium difficile-associated diarrhea., PMID:28837182
Epitopes and Mechanism of Action of the Clostridium difficile Toxin A-Neutralizing Antibody Actoxumab., PMID:28232034
Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection., PMID:28121498
The Monoclonal Antitoxin Antibodies (Actoxumab-Bezlotoxumab) Treatment Facilitates Normalization of the Gut Microbiota of Mice with Clostridium difficile Infection., PMID:27757389
Disease Progression and Resolution in Rodent Models of Clostridium difficile Infection and Impact of Antitoxin Antibodies and Vancomycin., PMID:27527088
Mechanisms of protection against Clostridium difficile infection by the monoclonal antitoxin antibodies actoxumab and bezlotoxumab., PMID:25486992
Antibodies to watch in 2015., PMID:25484055
Broad coverage of genetically diverse strains of Clostridium difficile by actoxumab and bezlotoxumab predicted by in vitro neutralization and epitope modeling., PMID:25451052
Toxin-mediated paracellular transport of antitoxin antibodies facilitates protection against Clostridium difficile infection., PMID:25385797
Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography., PMID:24821719
Which are the antibodies to watch in 2013?, PMID:23254906