Catalog No.
DGK07802
Expression system
Mammalian Cells
Species reactivity
General
Host species
Chimeric
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Ganglioside GD2
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
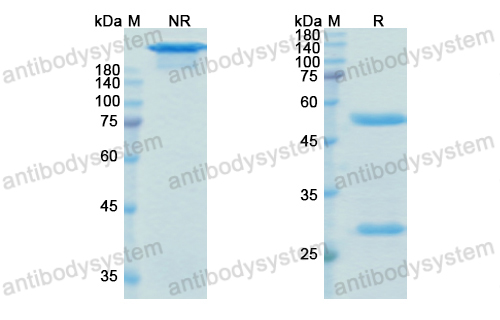
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
CAS: 65988-71-8
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
14.18 mAb, 14.18-IL-2, EMD 273063, EMD-273063, Hu14.18-IL-2, Hu14.18-IL2, anti-GD2 fused to IL2, hu14.18-IL2, hu14.18-Interleukin-2 fusion protein, CAS: 2131168-99-3
Clone ID
Lorukafusp Alfa
Safety and feasibility of an in situ vaccination and immunomodulatory targeted radionuclide combination immuno-radiotherapy approach in a comparative (companion dog) setting., PMID:34383787
Protection of hUC-MSCs against neuronal complement C3a receptor-mediated NLRP3 activation in CUMS-induced mice., PMID:33161108
Outcome-Related Signatures Identified by Whole Transcriptome Sequencing of Resectable Stage III/IV Melanoma Evaluated after Starting Hu14.18-IL2., PMID:32152202
Antitumor Activity and Tolerability of hu14.18-IL2 with GMCSF and Isotretinoin in Recurrent or Refractory Neuroblastoma: A Children's Oncology Group Phase II Study., PMID:31358541
Pilot trial of the hu14.18-IL2 immunocytokine in patients with completely resectable recurrent stage III or stage IV melanoma., PMID:30073390
Intratumoral treatment of smaller mouse neuroblastoma tumors with a recombinant protein consisting of IL-2 linked to the hu14.18 antibody increases intratumoral CD8+ T and NK cells and improves survival., PMID:23661160
Phase II trial of hu14.18-IL2 for patients with metastatic melanoma., PMID:22678096
Uterine leiomyosarcoma diffusely express disialoganglioside GD2 and bind the therapeutic immunocytokine 14.18-IL2: implications for immunotherapy., PMID:22562378
Comparison of GD2 binding capture ELISA assays for anti-GD2-antibodies using GD2-coated plates and a GD2-expressing cell-based ELISA., PMID:21893062
Differential internalization of hu14.18-IL2 immunocytokine by NK and tumor cell: impact on conjugation, cytotoxicity, and targeting., PMID:21248148
Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy., PMID:20935224
Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study., PMID:20921469
Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma., PMID:19737959
Phase I/II open-label study of the biologic effects of the interleukin-2 immunocytokine EMD 273063 (hu14.18-IL2) in patients with metastatic malignant melanoma., PMID:19640287
The development of antibody-IL-2 based immunotherapy with hu14.18-IL2 (EMD-273063) in melanoma and neuroblastoma., PMID:19548853
A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group., PMID:16551859
Technology evaluation: EMD-273063, EMD Lexigen., PMID:15537058
Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients., PMID:15483010