Catalog No.
DHB86906
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-nd
Clonality
Monoclonal
Target
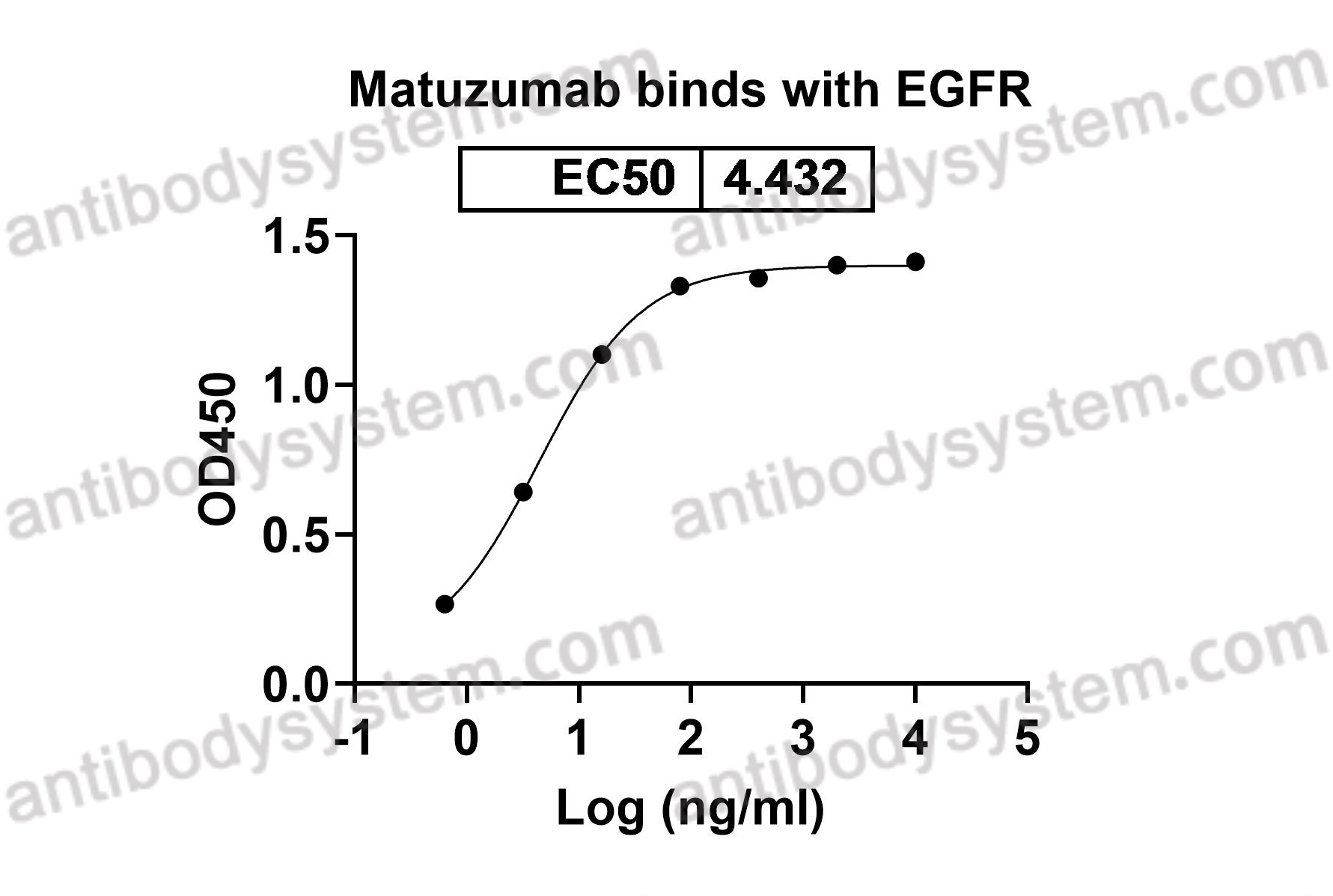
HER1, Receptor tyrosine-protein kinase erbB-1, Proto-oncogene c-ErbB-1, Epidermal growth factor receptor, EGFR, ERBB1, ERBB
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
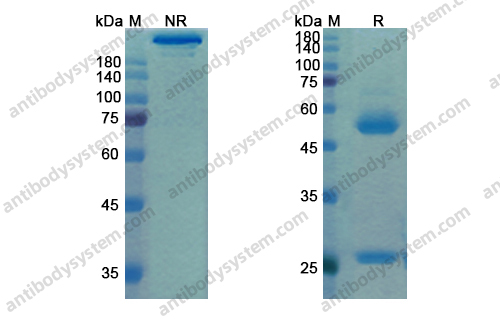
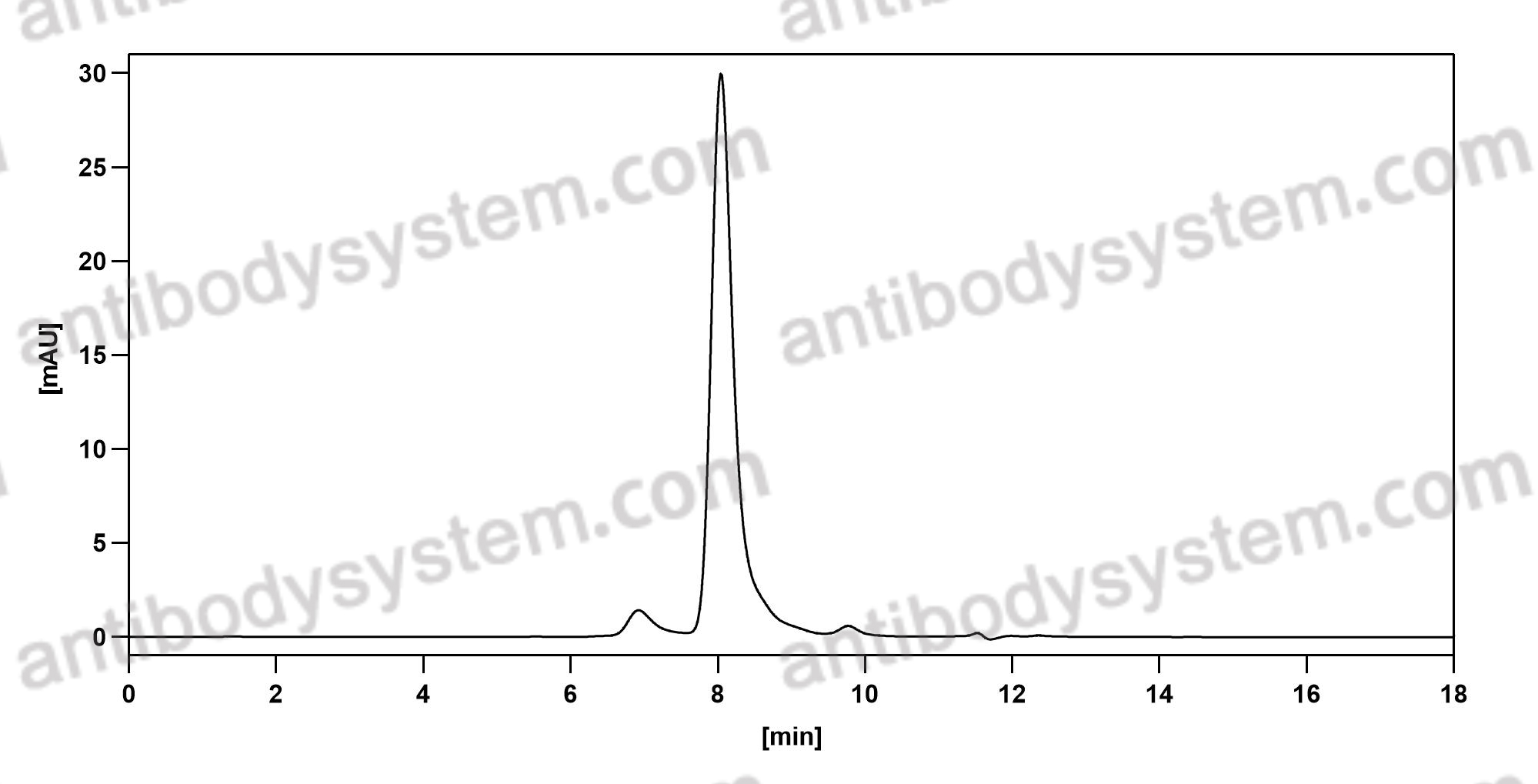
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P00533
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
EMD-72000, H425, h425, CAS: 339186-68-4
Clone ID
Matuzumab
An Anti-EGFR Antibody-Drug Radioconjugate Labeled with Actinium-225 Elicits Durable Antitumor Responses in KRAS- and BRAF-Mutant Colorectal Cancer., PMID:40126545
Evaluation of a cIEF Fractionation Workflow for Offline MS Analysis of Charge Variants of the Monoclonal Antibody Matuzumab., PMID:39964944
Enhancing Neutrophil Cytotoxicity of a Panel of Clinical EGFR Antibodies by Fc Engineering to IgA3.0., PMID:38958494
Pharmacophore-based virtual screening approaches to identify novel molecular candidates against EGFR through comprehensive computational approaches and in-vitro studies., PMID:36457709
New Perspectives for Prosthetic Valve Endocarditis: Impact of Molecular Imaging by FISHseq Diagnostics., PMID:36318608
Simultaneous Imaging and Therapy Using Epitope-Specific Anti-Epidermal Growth Factor Receptor (EGFR) Antibody Conjugates., PMID:36145664
Milking the Cow: Cattle-Derived Chimeric Ultralong CDR-H3 Antibodies and Their Engineered CDR-H3-Only Knobbody Counterparts Targeting Epidermal Growth Factor Receptor Elicit Potent NK Cell-Mediated Cytotoxicity., PMID:34759924
Diverse metabolic response of cancer cells treated with a 213Bi-anti-EGFR-immunoconjugate., PMID:33737524
Identification of a novel anti-EGFR nanobody by phage display and its distinct paratope and epitope via homology modeling and molecular docking., PMID:33130376
Ultrabright Terbium Nanoparticles for FRET Biosensing and in Situ Imaging of Epidermal Growth Factor Receptors*., PMID:32501573
Improving antibody-based therapies by chemical engineering of antibodies with multimeric cell-penetrating peptides for elevated intracellular delivery., PMID:32184098
The effects of somatic mutations on EGFR interaction with anti-EGFR monoclonal antibodies: Implication for acquired resistance., PMID:31228284
Most clinical anti-EGFR antibodies do not neutralize both wtEGFR and EGFRvIII activation in glioma., PMID:31002307
Risk of fatigue in cancer patients receiving anti-EGFR monoclonal antibodies: results from a systematic review and meta-analysis of randomized controlled trial., PMID:29181651
Semi-synthetic vNAR libraries screened against therapeutic antibodies primarily deliver anti-idiotypic binders., PMID:28852148
Evaluation of short-term effectiveness of eight targeted agents combined with chemotherapy for treating esophageal-gastric junction adenocarcinoma: A network meta-analysis., PMID:28708307
[Research status quo and progression in targeted therapy for advanced gastric cancer]., PMID:27781258
A Complement-Optimized EGFR Antibody Improves Cytotoxic Functions of Polymorphonuclear Cells against Tumor Cells., PMID:26475927
²¹³Bi-anti-EGFR radioimmunoconjugates and X-ray irradiation trigger different cell death pathways in squamous cell carcinoma cells., PMID:24210808
Structural evaluation of EGFR inhibition mechanisms for nanobodies/VHH domains., PMID:23791944
Alpha-particle emitting 213Bi-anti-EGFR immunoconjugates eradicate tumor cells independent of oxygenation., PMID:23724085
[A perspective: role of targeted therapy in colon cancer]., PMID:23575231
EGFR-directed monoclonal antibodies in non-small cell lung cancer., PMID:23300028
A phase I pharmacokinetic study of matuzumab in combination with paclitaxel in patients with EGFR-expressing advanced non-small cell lung cancer., PMID:22832803
Metastatic gastric cancer - focus on targeted therapies., PMID:22807624
Phase I study of matuzumab in combination with 5-fluorouracil, leucovorin and cisplatin (PLF) in patients with advanced gastric and esophagogastric adenocarcinomas., PMID:22763610
Anti-EGFR biparatopic-SEED antibody has enhanced combination-activity in a single molecule., PMID:22426455
In situ analysis of mutant EGFRs prevalent in glioblastoma multiforme reveals aberrant dimerization, activation, and differential response to anti-EGFR targeted therapy., PMID:22232519
Efficient blockade of Akt signaling is a determinant factor to overcome resistance to matuzumab., PMID:22185378
In-depth biophysical analysis of interactions between therapeutic antibodies and the extracellular domain of the epidermal growth factor receptor., PMID:22085444
A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth., PMID:21520037
Targeted signal-amplifying enzymes enhance MRI of EGFR expression in an orthotopic model of human glioma., PMID:21245103
Anti-EGFR monoclonal antibody in cancer treatment: in vitro and in vivo evidence., PMID:21196277
Monoclonal antibodies against EGFR in non-small cell lung cancer., PMID:21109448
Pemetrexed with or without matuzumab as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer., PMID:20978446
Peptide mimotopes recognized by antibodies cetuximab and matuzumab induce a functionally equivalent anti-EGFR immune response., PMID:20514015
Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study., PMID:20497967
[The current status of development of anti-EGFR antibodies]., PMID:20495305
Refinement of the population pharmacokinetic model for the monoclonal antibody matuzumab: external model evaluation and simulations., PMID:19691369
Cetuximab and other anti-epidermal growth factor receptor monoclonal antibodies in the treatment of non-small cell lung cancer., PMID:19482958
Interaction between fatty acid synthase- and ErbB-systems in ovarian cancer cells., PMID:19467222
Extending outcomes: epidermal growth factor receptor-targeted monoclonal antibodies in non-small-cell lung cancer., PMID:19362943
Molecular determinants of response to matuzumab in combination with paclitaxel for patients with advanced non-small cell lung cancer., PMID:19276157
Anti-epidermal growth factor receptor monoclonal antibodies in cancer treatment., PMID:19269105
Phase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancer., PMID:19238629
A novel mechanism for anti-EGFR antibody action involves chemokine-mediated leukocyte infiltration., PMID:19208382
Different antiproliferative effects of matuzumab and cetuximab in A431 cells are associated with persistent activity of the MAPK pathway., PMID:19167213
[Targeting HER pathway in head and neck and thoracic cancers]., PMID:19433372
The anti-EGFR monoclonal antibody blocks cisplatin-induced activation of EGFR signaling mediated by HB-EGF., PMID:19027738
The role of cetuximab and other epidermal growth factor receptor monoclonal antibodies in the treatment of advanced non-small cell lung cancer., PMID:18782080