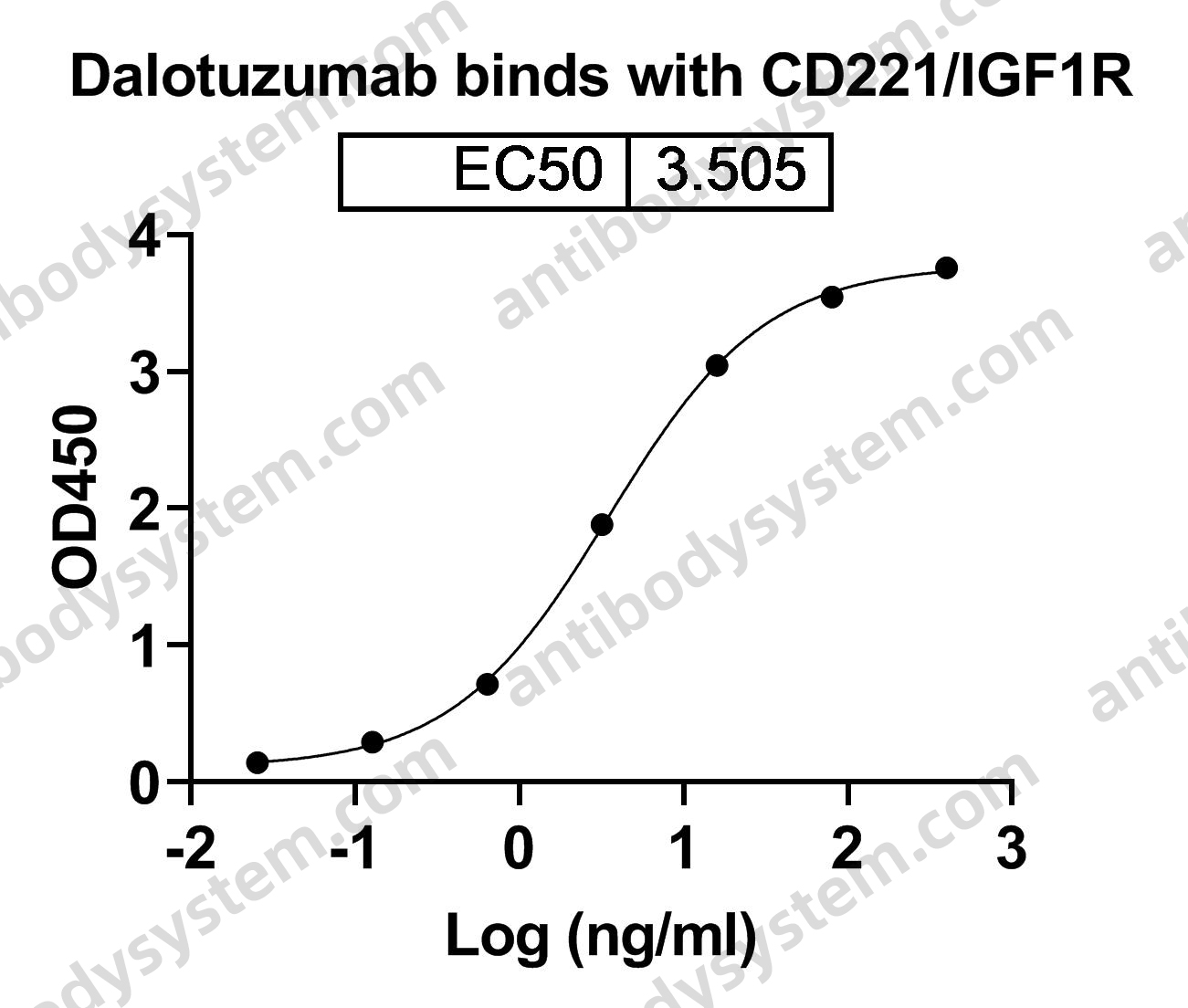
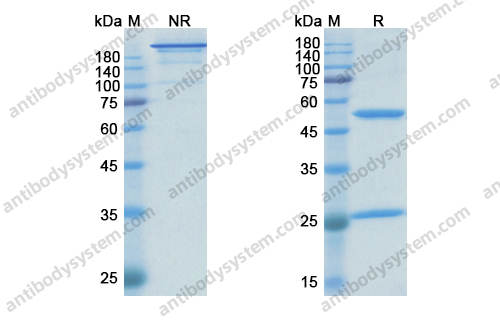
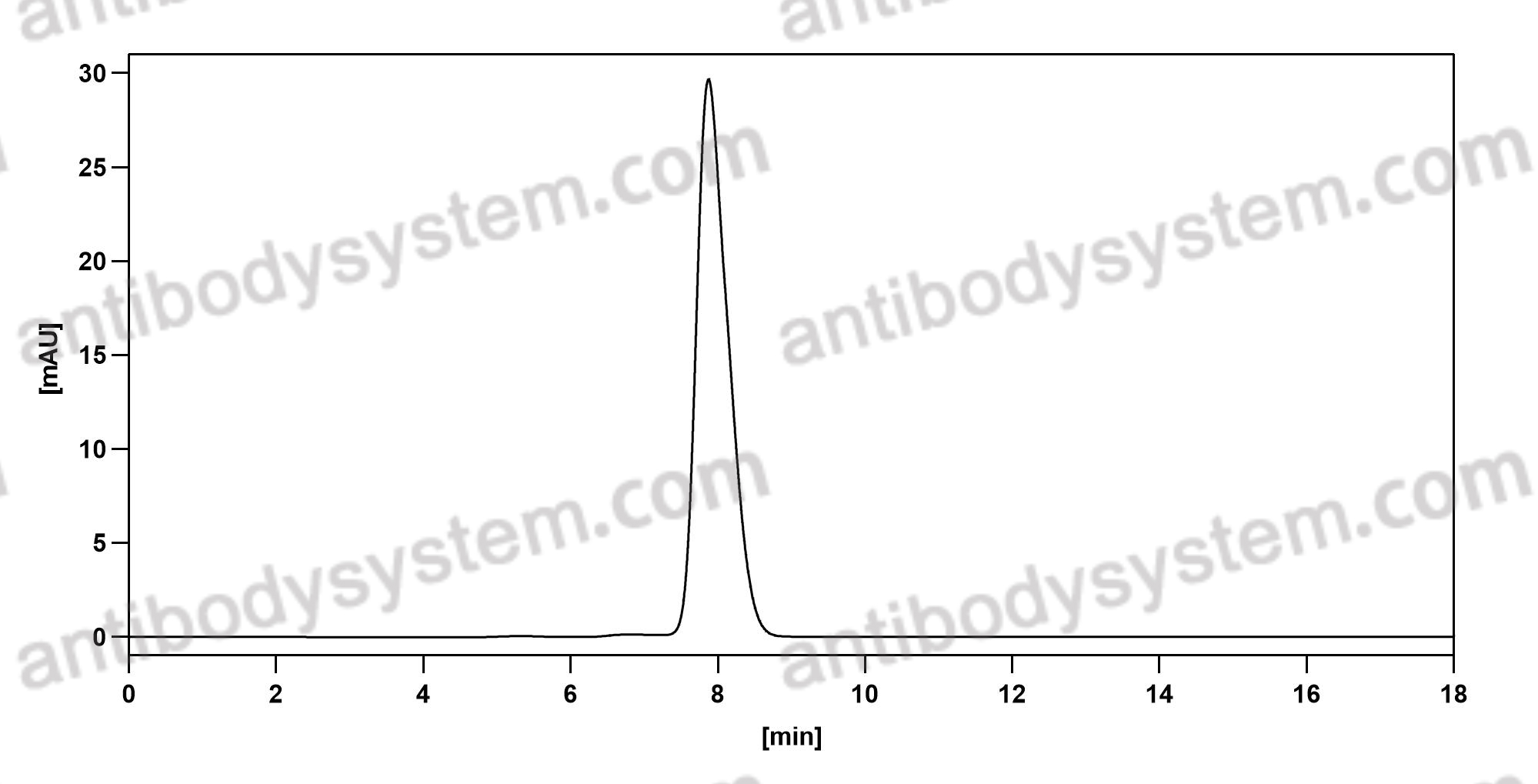
Catalog No.
DHC29901
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
CD221, Insulin-like growth factor 1 receptor, IGF1R, Insulin-like growth factor I receptor, IGF-I receptor
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P08069
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MK-0646, CAS: 1005389-60-5
Clone ID
Dalotuzumab
Employing splice-switching oligonucleotides and AAVrh74.U7 snRNA to target insulin receptor splicing and cancer hallmarks in osteosarcoma., PMID:39720325
The efficacy of targeted therapy and/or immunotherapy with or without chemotherapy in patients with colorectal cancer: A network meta-analysis., PMID:39716565
Clinical research progress of ridaforolimus (AP23573, MK8668) over the past decade: a systemic review., PMID:38584599
Randomized, phase I/II study of gemcitabine plus IGF-1R antagonist (MK-0646) versus gemcitabine plus erlotinib with and without MK-0646 for advanced pancreatic adenocarcinoma., PMID:29843755
Determination of isoelectric points and relative charge variants of 23 therapeutic monoclonal antibodies., PMID:28961486
A randomized phase II trial of ridaforolimus, dalotuzumab, and exemestane compared with ridaforolimus and exemestane in patients with advanced breast cancer., PMID:28681171
Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy., PMID:28427155
A phase II study of combined ridaforolimus and dalotuzumab compared with exemestane in patients with estrogen receptor-positive breast cancer., PMID:28324268
Ridaforolimus (MK-8669) synergizes with Dalotuzumab (MK-0646) in hormone-sensitive breast cancer., PMID:27765027
Broad RTK-targeted therapy overcomes molecular heterogeneity-driven resistance to cetuximab via vectored immunoprophylaxis in colorectal cancer., PMID:27569653
Dalotuzumab in chemorefractory KRAS exon 2 mutant colorectal cancer: Results from a randomised phase II/III trial., PMID:27681944
IGF-1R and mTOR Blockade: Novel Resistance Mechanisms and Synergistic Drug Combinations for Ewing Sarcoma., PMID:27576731
Physiologically-based pharmacokinetic modeling to predict the clinical pharmacokinetics of monoclonal antibodies., PMID:27377311
Emerging antibodies for the treatment of multiple myeloma., PMID:27195659
Phase 1 study of dalotuzumab monotherapy and ridaforolimus-dalotuzumab combination therapy in paediatric patients with advanced solid tumours., PMID:27185573
Impact Study: MK-0646 (Dalotuzumab), Insulin Growth Factor 1 Receptor Antibody Combined with Pemetrexed and Cisplatin in Stage IV Metastatic Non-squamous Lung Cancer., PMID:26793618
RE: A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer., PMID:26754849
Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade., PMID:26429980
A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer., PMID:26405092
Minireview: Were the IGF Signaling Inhibitors All Bad?, PMID:26366975
Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma., PMID:26240353
Activity of dalotuzumab, a selective anti-IGF1R antibody, in combination with erlotinib in unselected patients with Non-small-cell lung cancer: a phase I/II randomized trial., PMID:25414803
Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: preclinical characterization and phase I clinical trial., PMID:25320355
A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours., PMID:25290091
Targeting tyrosine-kinases and estrogen receptor abrogates resistance to endocrine therapy in breast cancer., PMID:24979294
NCIC CTG IND.190 phase I trial of dalotuzumab (MK-0646) in combination with cisplatin and etoposide in extensive-stage small-cell lung cancer., PMID:24518092
Ezrin expression and cell survival regulation in colorectal cancer., PMID:24462708
In vivo analysis of insulin-like growth factor type 1 receptor humanized monoclonal antibody MK-0646 and small molecule kinase inhibitor OSI-906 in colorectal cancer., PMID:24173770
The adverse events profile of anti-IGF-1R monoclonal antibodies in cancer therapy., PMID:24033707
Phase 1 pharmacokinetic study of MK-0646 (dalotuzumab), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in combination with cetuximab and irinotecan in Japanese patients with advanced colorectal cancer., PMID:23921573
Trial watch: Monoclonal antibodies in cancer therapy., PMID:23482847
IGF1R-directed targeted therapy enhances the cytotoxic effect of chemotherapy in endometrial cancer., PMID:23402816
Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment., PMID:22573715
A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors., PMID:22437754
Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment., PMID:22042973
Evaluation of two ELISA methods to detect therapeutic anti-IGF1R antibodies in clinical study samples of dalotuzumab., PMID:21942521
The mechanism of action of the histone deacetylase inhibitor vorinostat involves interaction with the insulin-like growth factor signaling pathway., PMID:21931726
The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors., PMID:21907495
A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors., PMID:21810918
Antibody-based therapeutics to watch in 2011., PMID:21051951
Dalotuzumab, a recombinant humanized mAb targeted against IGFR1 for the treatment of cancer., PMID:20521225