Catalog No.
DHH28808
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
VH-VH'-VH
Clonality
Monoclonal
Target
CTLA-8, Cytotoxic T-lymphocyte-associated antigen 8, IL17, IL-17A, IL17A, CTLA8, IL-17, Interleukin-17A, IL17F, Interleukin-17F, IL-17F, Cytokine ML-1, ALB, Albumin
Concentration
1.42 mg/ml
Endotoxin level
Please contact with the lab for this information.
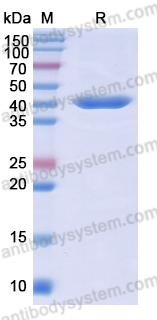
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q16552 & Q96PD4 & P02768
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
30 mM histidine pH 5.8,10% sucrose, 0.02% Tween 80
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Bispecific, Trivalent, M-1095, MSB-0010841, CAS: 1414386-05-2
Clone ID
Sonelokimab
Assessing the clinical progress of the bispecific nanobody sonelokimab., PMID:40297934
Next-Generation Anti-IL-17 Agents for Psoriatic Disease: A Pipeline Review., PMID:39982633
Interleukin-17: A pleiotropic cytokine implicated in inflammatory, infectious, and malignant disorders., PMID:39875232
Antibodies to watch in 2025., PMID:39711140
Interleukin-17 Inhibitors in the Treatment of Hidradenitis Suppurativa., PMID:39604776
IL-17 Inhibition: A Valid Therapeutic Strategy in the Management of Hidradenitis Suppurativa., PMID:37896210
Pathogenic Role of IL-17 and Therapeutic Targeting of IL-17F in Psoriatic Arthritis and Spondyloarthropathies., PMID:37373452
Comparation of time-course, dose-effect, influencing factors and adverse events of biologics in the treatment of adults with moderate to severe plaque psoriasis., PMID:37304299
Biological treatment for erythrodermic psoriasis., PMID:36154361
Maintenance of response in moderate-to-severe psoriasis after withdrawal of the interleukin (IL)-17A and IL-17F nanobody sonelokimab: is there a role for IL-17F in disease reoccurrence?, PMID:35442535
Dual inhibition of IL-17A and IL-17F in psoriatic disease., PMID:34408825
Latest Advances for the Treatment of Chronic Plaque Psoriasis with Biologics and Oral Small Molecules., PMID:34239295
IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomised, placebo-controlled, phase 2b study., PMID:33894834