Decoding the Antibody Therapeutics Landscape: Four Pillars from 209 Approved Drugs
Antibody therapeutics have become one of the most successful classes of biologic drugs in modern medicine. Over the past two decades, they have fundamentally reshaped the treatment landscape for cancer, autoimmune diseases, and beyond.
Yet, for those working in antibody discovery, development, or manufacturing, it is increasingly clear that clinical success is not determined by target selection alone. Molecular format, functional design, and—critically—how an antibody is manufactured all play decisive roles in determining whether a candidate ultimately reaches patients.
 A recent comprehensive study published in The AAPS Journal provides a rare, panoramic view of this field. By systematically analyzing 209 therapeutic antibodies approved by the U.S. FDA up to May 2025, the authors map the antibody universe across four fundamental dimensions: therapeutic indication, molecular format, IgG subclass, and expression system.
A recent comprehensive study published in The AAPS Journal provides a rare, panoramic view of this field. By systematically analyzing 209 therapeutic antibodies approved by the U.S. FDA up to May 2025, the authors map the antibody universe across four fundamental dimensions: therapeutic indication, molecular format, IgG subclass, and expression system.
Together, these dimensions form a structural framework that explains not only where the field stands today, but also where it is heading
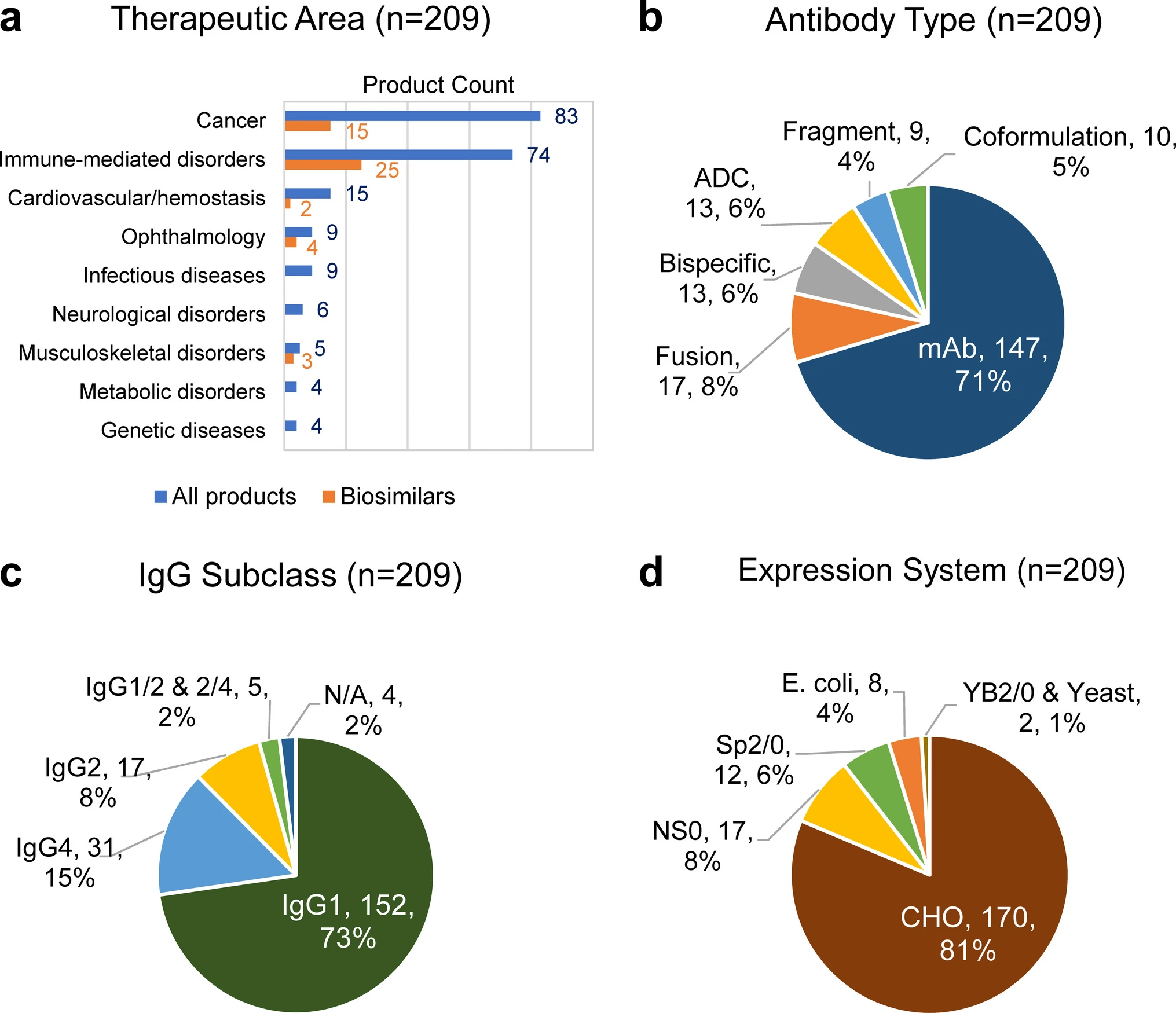
Overview of FDA-approved therapeutic antibodies. Categorization of FDA-approved therapeutic antibodies (n = 209; as of May 2025) by a clinical indication, b antibody type, c IgG subclass, and d expression system
Pillar I: Therapeutic Focus
Oncology and Autoimmune Diseases as the Central Arenas
Antibody drug development closely follows areas of the highest unmet medical need—and the approved portfolio reflects this clearly.
Among the 209 FDA-approved antibodies, nearly 40% (83 drugs) target oncology indications, making cancer the single largest therapeutic area. From early landmark antibodies such as rituximab and trastuzumab to more recent immune checkpoint inhibitors and antibody–drug conjugates (ADCs), antibodies have become a cornerstone of modern cancer therapy.
The second major domain is autoimmune and inflammatory diseases, accounting for 74 approved antibodies. Therapeutics targeting TNF-α, IL-6R, IL-17/23, and related pathways have transformed the management of chronic immune-mediated disorders, including rheumatoid arthritis, psoriasis, and inflammatory bowel disease.
Beyond these two dominant fields, antibodies have also secured positions in cardiovascular diseases (15 drugs), infectious diseases (9 drugs), and other indications. Overall, the distribution reveals a clear "dual-core" strategy, with oncology and autoimmune diseases acting as the primary gravitational centers of antibody innovation.
Pillar II: Molecular Formats
Monoclonal Antibodies as the Foundation, Innovation at the Edges
From a structural perspective, antibody therapeutics are far from homogeneous.
Conventional monoclonal antibodies (mAbs) remain the backbone of the field, representing 71% (147 drugs) of all approved products. Their well-characterized structure, predictable pharmacology, and established manufacturing processes make them the most reliable and widely adopted format.
At the same time, advanced formats such as ADCs and bispecific antibodies (BsAbs) are rapidly gaining momentum, each accounting for approximately 6% (13 drugs) of approvals. ADCs combine the targeting specificity of antibodies with highly potent cytotoxic payloads, enabling selective tumor cell killing. Bispecific antibodies, by simultaneously engaging two distinct targets, have opened new therapeutic paradigms—particularly in immuno-oncology.
Additional formats, including Fc-fusion proteins (8%, 17 drugs), antibody fragments (4%, 9 drugs), and fixed-dose combinations (5%, 10 drugs), further expand the functional and clinical versatility of antibody-based therapies.
This diversification of molecular formats highlights the field's ongoing effort to optimize efficacy, safety, and patient convenience through structural innovation.
Pillar III: Functional Design
IgG Subclass Selection Shapes Immune Engagement
At the molecular level, the IgG subclass of an antibody is a critical determinant of its interaction with the immune system.
Among approved antibodies, IgG1 overwhelmingly dominates, accounting for 73% of products. Its Fc region efficiently mediates immune effector functions such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), making IgG1 the preferred choice when elimination of target cells—such as tumor cells—is desired.
In contrast, IgG4 antibodies represent approximately 15% of approvals. With markedly reduced effector functions, IgG4 is well suited for applications where neutralization or pathway blockade is required without inducing cell killing. This property explains its frequent use in autoimmune and inflammatory disease indications.
The resulting pattern—IgG1 for cytotoxic clearance, IgG4 for functional blockade—reflects a highly rational, mechanism-driven approach to antibody design.
Pillar IV: Manufacturing Platforms
Expression Systems as the Invisible Determinant of Success
Behind every approved antibody lies a production system capable of delivering consistent quality at commercial scale.
In this regard, the data reveal a clear industry consensus. CHO (Chinese hamster ovary) cells dominate antibody manufacturing, accounting for approximately 81% of approved products. The primary reason is their ability to produce human-like glycosylation profiles—a critical quality attribute that directly affects antibody stability, pharmacokinetics, and immunogenicity.
Earlier-generation murine myeloma cell lines, such as NS0 (8%) and Sp2/0 (6%), are still represented among approved products but have gradually fallen out of favor. These systems can introduce non-human glycan structures (e.g., α-Gal, NGNA), which have been associated with adverse immune reactions. The well-documented case of cetuximab, expressed in Sp2/0 cells and linked to α-Gal–mediated hypersensitivity in certain patients, underscores the clinical relevance of expression system choice.
Notably, a small number of antibody fragments have been produced using Escherichia coli, reflecting situations where Fc-mediated functions and glycosylation are not required. Yeast-based systems, while valuable for certain recombinant proteins, remain rare among FDA-approved therapeutic antibodies to date.
Ultimately, expression system selection represents a strategic balance between molecular complexity, safety, manufacturability, and cost.
Concluding Perspective
One Figure, Four Pillars, and the Logic of an Industry
Viewed together, these 209 FDA-approved antibodies reveal a remarkably coherent structure underpinning the antibody therapeutics industry:
- A therapeutic focus centered on oncology and autoimmune diseases
- A foundation of monoclonal antibodies complemented by emerging formats
- IgG subclass selection aligned with functional intent
- And CHO-based manufacturing as the prevailing standard for quality and safety
This landscape is not static. As antibody architectures become increasingly complex and clinical expectations continue to rise, control over expression systems, glycosylation patterns, and other critical quality attributes will play an even more central role in determining long-term success.
As antibody therapeutics advance toward more complex modalities such as ADCs and bispecific antibodies, antibody quality attributes—including structural integrity and functional consistency—are becoming critical determinants of development success.
Aligned with these industry trends, AntibodySystem offers research-grade monoclonal antibodies, bispecific antibody, and ADC-related reagents to support antibody discovery, engineering, and functional studies.
| Catalog | Product Name |
|---|---|
| PHH02201 | Anti-Human CD279/PDCD1/PD1 Polyclonal Antibody |
| DHC43399 | Datopotamab deruxtecan (ADC) |
| VHH02202 | InVivoMAb Anti-Human CD279/PDCD1/PD1 Antibody (Iv0209) |
| VMH02204 | InVivoMAb Anti-Mouse PD-1 & PD-L1 Bispecific Antibody (Iv0240) |
| DHC21097 | Labetuzumab govitecan (ADC) |
| DHB65499 | Lifastuzumab vedotin (ADC) |
| DHH02221 | Research Grade Cadonilimab |
| DHC90713 | Research Grade Epcoritamab |
| DHC90714 | Research Grade Glofitamab |
| DHC90716 | Research Grade Mosunetuzumab |
| DHJ70105 | Research Grade Pacmilimab |
| DHD30407 | Research Grade Tebotelimab |
| DHC21099 | Tusamitamab ravtansine (ADC) |
| DHC21098 | Tusamitamab-MMAE (ADC) |
Therapeutic Targets & Antibodies
