In September 2025, a research paper titled "Targeting necrotic lipid release in tumors enhances immunosurveillance and cancer immunotherapy of glioblastoma" in the journal Cell Research. This study reveals for the first time a novel immune evasion mechanism whereby loss of pro-apoptotic tumor suppressor genes (such as TP53) triggers mPTP-dependent necrosis, releasing APOE-containing lipid particles that lead to T cell dysfunction. Furthermore, it proposes that clearing excess APOE lipid particles from the immune microenvironment is a new strategy to restore the function of tumor-infiltrating T cells and enhance the efficacy of immunotherapy for glioblastoma (GBM).

AntibodySystem Product
This study utilized AntibodySystem's Anti-Mouse APOE Antibody (HJ6.3) (Catalog #RMB98601), delivered via mini-osmotic pumps for localized intracranial administration in a Trp53-mutated mouse GBM model. The research found that the antibody alone, by neutralizing APOE lipid particles released from necrotic tumor regions, significantly prolonged mouse survival. Its mechanism of action differs from immune checkpoint inhibitors; rather than directly enhancing T cell killing capacity, it works by inhibiting lipid accumulation and apoptosis in T cells, thereby "rescuing" dysfunctional T cells. More importantly, when this antibody was combined with an anti-PD-1 antibody, it demonstrated a powerful synergistic effect, providing a new strategy for GBM immunotherapy.
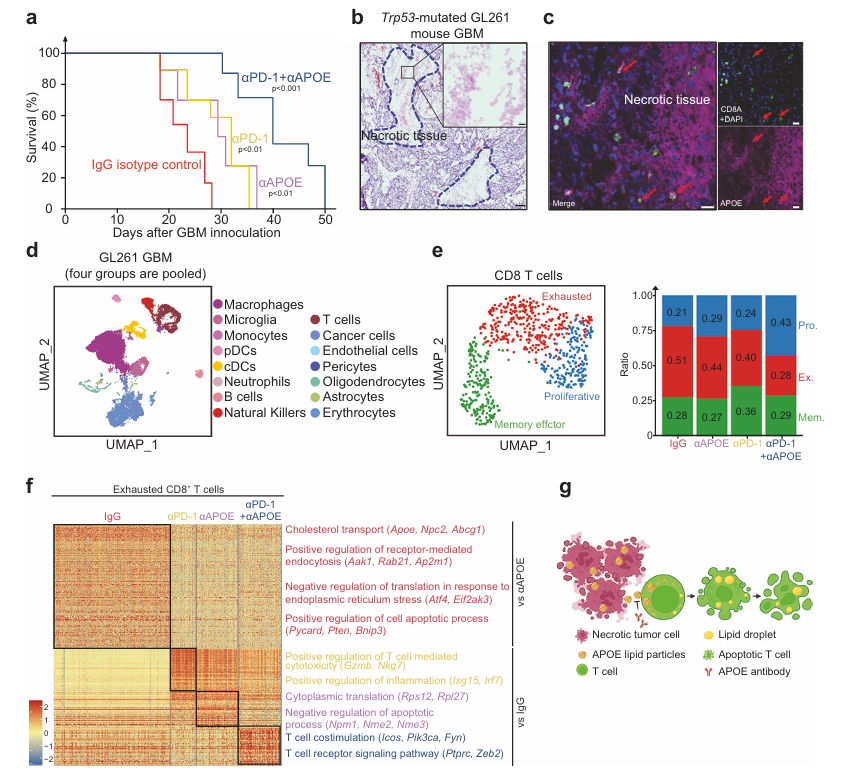
Figure 1. APOE and PD-1 antibodies boost anti-tumor immunity in mouse GBM model
Background
GBM is the most common and aggressive primary brain tumor in adults, with limited advancements in treatment and typically poor prognosis. Although surgical resection, radiotherapy, and chemotherapy are standard treatments, most patients have short survival times and limited therapeutic efficacy. The immune microenvironment of GBM is particularly complex, with tumor cells employing various mechanisms to suppress immune responses and evade immune surveillance. Recent studies have revealed that tumor necrosis is a hallmark pathological feature of GBM, but its specific role in immune evasion remains unclear.
The cancer immunoediting theory describes a dynamic three-phase process of interaction between the immune system and tumors: elimination, equilibrium, and escape. Most current research focuses on the "escape" phase, investigating how established tumors suppress the immune system. However, how tumors evade immune surveillance during the initial stages (i.e., the transition from "elimination" to "equilibrium") is still not well understood. This is primarily because commonly used syngeneic mouse tumor models employ established cell lines that have already undergone immunoediting during long-term in vitro culture and in vivo passaging; their genomic and immune characteristics do not represent the state during initial tumorigenesis.
To overcome this limitation, this study innovatively utilized mouse embryonic stem cells (mESCs) to construct a syngeneic teratoma model. The unique advantage of this model lies in the use of non-cancerous, immunologically naive mESCs, enabling the simulation of interactions between the immune system and nascent "tumor" cells during early tumorigenesis. Through a genome-wide CRISPR screen in this model, the researchers discovered that loss of pro-apoptotic tumor suppressor genes (such as Trp53 and Pten) is a key event driving early immune evasion.
Research Findings
Researchers analyzed key genes affecting early immune surveillance using a genome-wide CRISPR screening system. As shown in Figure 2a, the Brie CRISPR library was transduced into mESCs, which were then transplanted into immunocompetent or immunodeficient mice. Gene ranking plots (Fig. 2b-c) showed that sgRNAs targeting Trp53, Pten, Usp28, and Tp53bp1 were significantly enriched in immunocompetent mice, while sgRNAs targeting Bbc3 and Bax also showed selective enrichment. Growth curves (Fig. 2d-g) further confirmed that Trp53⁻/⁻, Pten⁻/⁻, Bbc3⁻/⁻, and Bax⁻/⁻ mESCs formed teratomas much more efficiently in immunocompetent mice, whereas the differences were not significant in immunodeficient mice. These results indicate that the loss of pro-apoptotic tumor suppressor genes selectively promotes tumor growth in immunocompetent hosts.
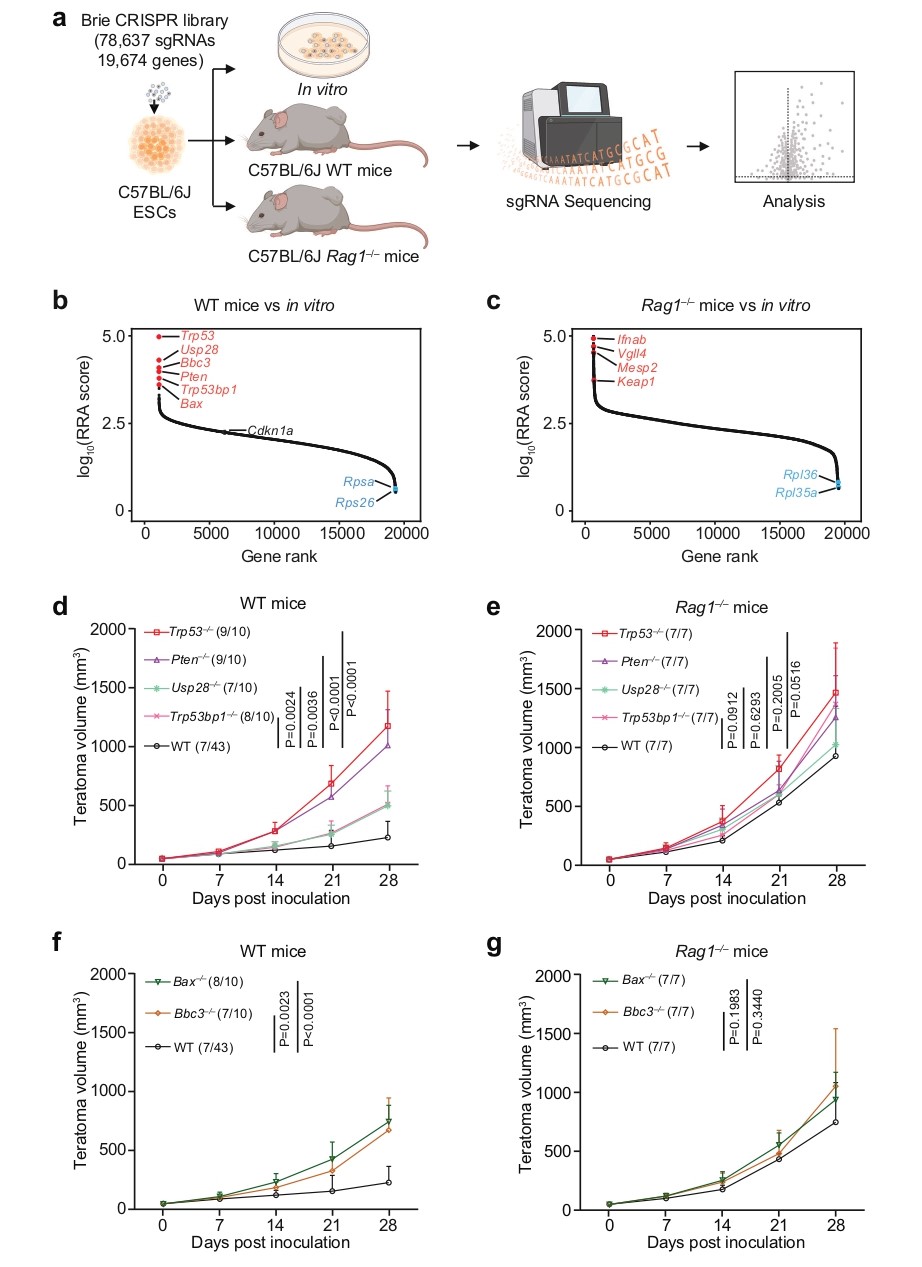
Figure 2. Genome-wide CRISPR screen for immunosurveillance genes
At the mechanistic level, the study found that Trp53-deficient teratomas exhibited significantly larger necrotic areas. Single-cell RNA sequencing analysis (Fig. 3a-e) revealed abnormal states of infiltrating T cells in Trp53⁻/⁻ teratomas: these T cells not only expressed exhaustion markers such as Pdcd1 and Lag3 but also upregulated genes related to endoplasmic reticulum stress and lipid metabolism. Immunohistochemistry and flow cytometry results (Fig. 3f-i) showed that T cells in Trp53⁻/⁻ teratomas had significantly increased levels of the lipid-droplet-associated protein PLIN2 and cholesteryl esters, along with elevated levels of the apoptosis markers cleaved Caspase-3 and cleaved PARP1, indicating that these cells were in a state of lipid accumulation and dysfunction.
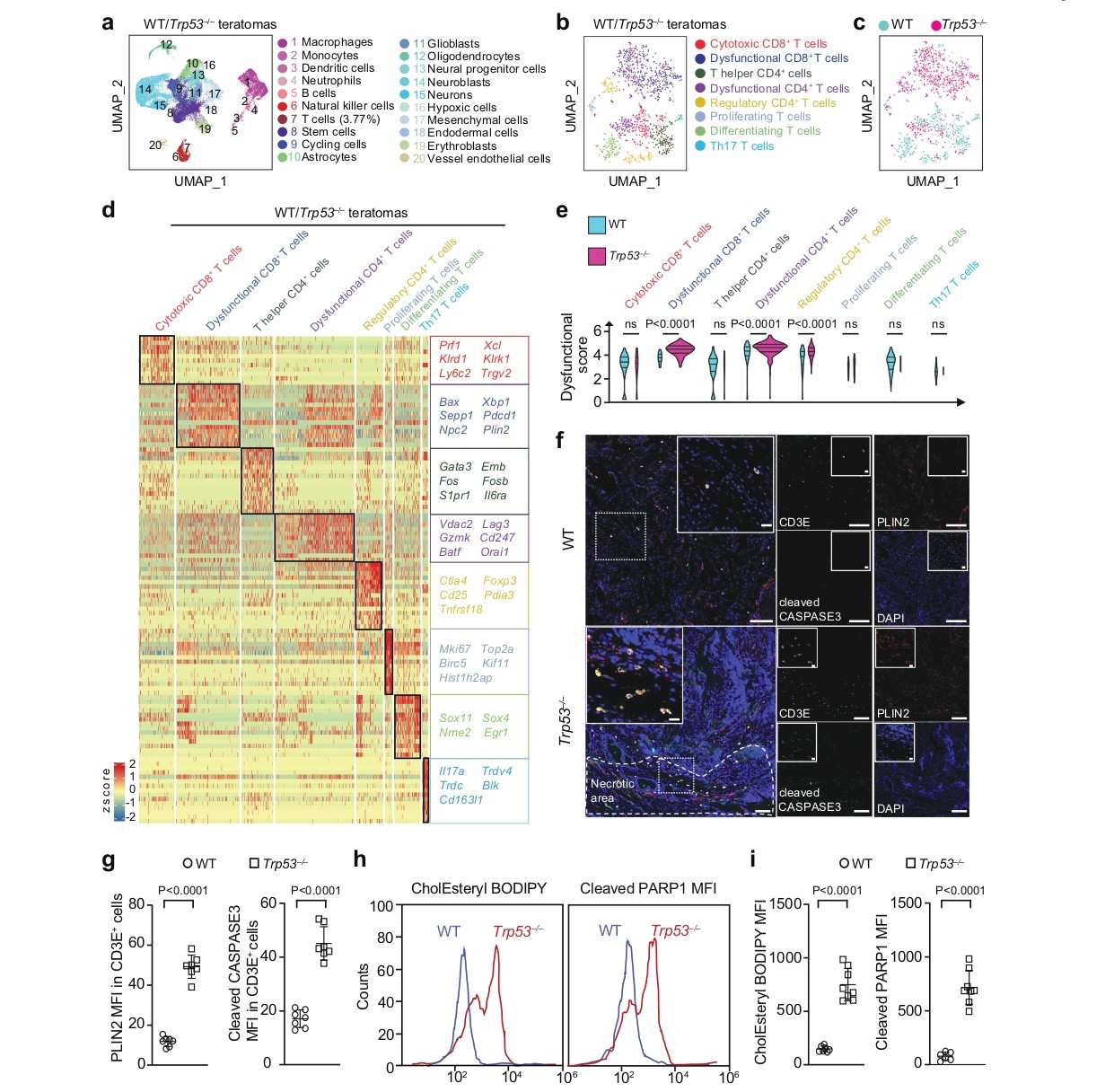
Figure 3. Profiling in filtrating Tcells in teratomas by scRNA-seq
Figure 4a-f further demonstrates a similar phenotype in tumor-associated macrophages (TAMs). Macrophages in Trp53⁻/⁻ teratomas upregulated pathways involved in the response to lipoprotein particles and intrinsic apoptotic signaling, while downregulating processes like mononuclear cell proliferation and glycolysis. Immunohistochemical staining showed that these macrophages also exhibited elevated levels of PLIN2 and cleaved Caspase-3, displaying a morphology reminiscent of foam cells in atherosclerotic plaques. These findings indicate that APOE-containing lipid particles released by necrotic tumor cells have a broad suppressive effect on various anti-tumor immune cells, including T cells and macrophages.
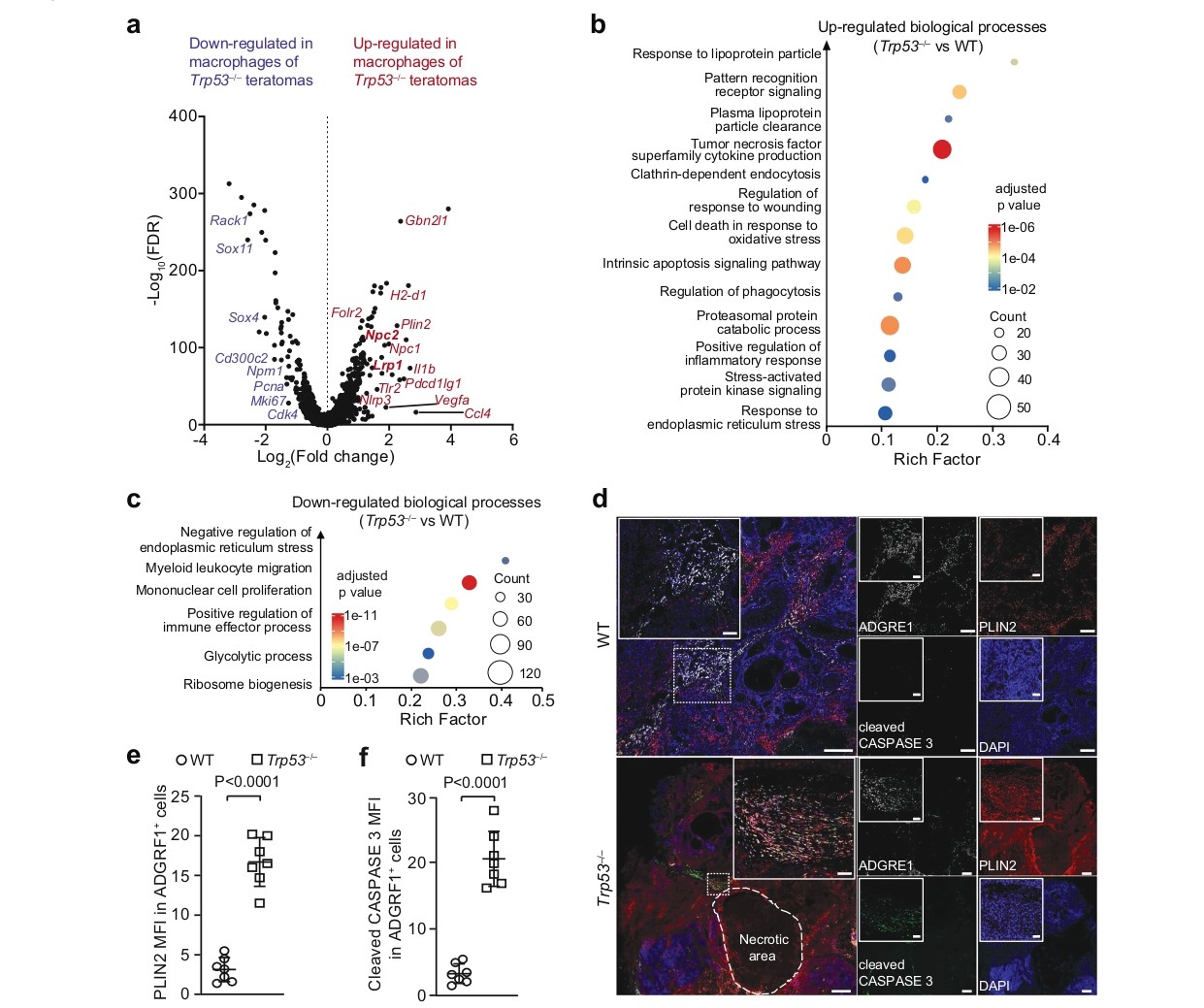
Figure 4. Lipid accumulation an dapoptosis of macrophages in Trp53–/– teratomas
To investigate the mechanism by which loss of pro-apoptotic genes leads to immune evasion, researchers first observed large necrotic areas in Trp53–/– teratomas (Fig. 5a-b). Further lipidomics analysis showed that the interstitial fluid from these teratomas contained significantly higher levels of various lipids, particularly polyunsaturated phospholipids, cholesteryl esters, and triacylglycerols (Fig. 5a). Immunohistochemistry confirmed the presence of abundant extracellular APOE in necrotic areas, and both T cells and macrophages infiltrating these regions showed strong APOE uptake (Fig. 5b-f). These results directly link tumor necrosis, APOE-containing lipid particle release, and lipid uptake by immune cells. It is noteworthy that this phenomenon of lipid accumulation and dysfunction was observed not only in T cells but also in tumor-associated macrophages.
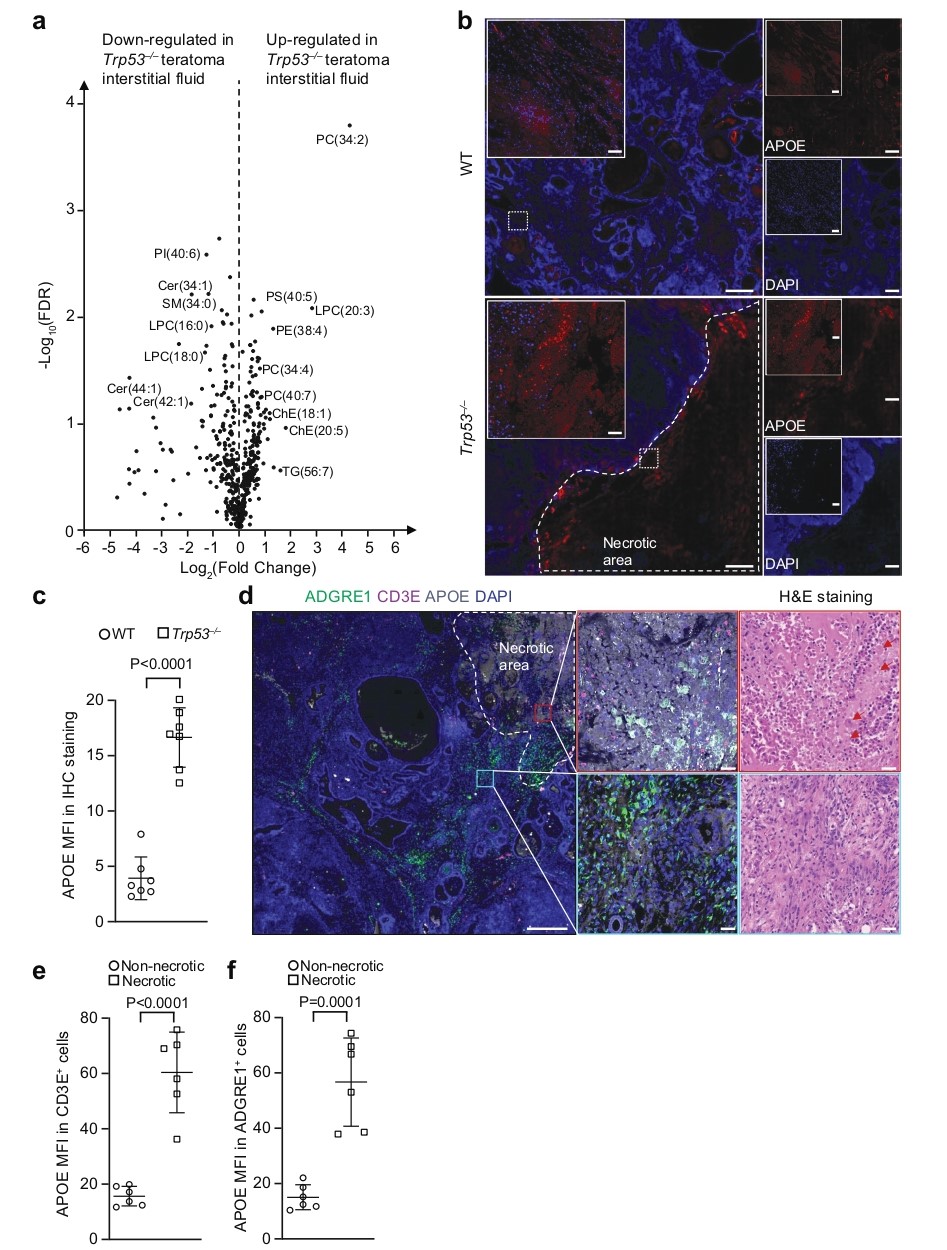
Figure 5. Necrotic teratoma cells release APOE lipid particles into tumor microenvironment
Could blocking necrosis reverse immune evasion? Researchers constructed a quintuple knockout (QKO) ESC line targeting core components of the mPTP. The results showed that QKO significantly reduced the necrotic area and the level of APOE in the interstitial fluid of Trp53⁻/⁻ teratomas (Fig. 6a-c, f). More importantly, this restored T cell function, significantly reducing their cholesteryl ester content and apoptosis levels (Fig. 6g-h). To confirm the specificity of this necrotic cell death pathway, researchers included a crucial control: knockout of the key necroptosis protein MLKL failed to produce the same effect, thereby excluding the involvement of the necroptosis pathway and demonstrating that mPTP-dependent necrosis is the critical step in this immune evasion mechanism.
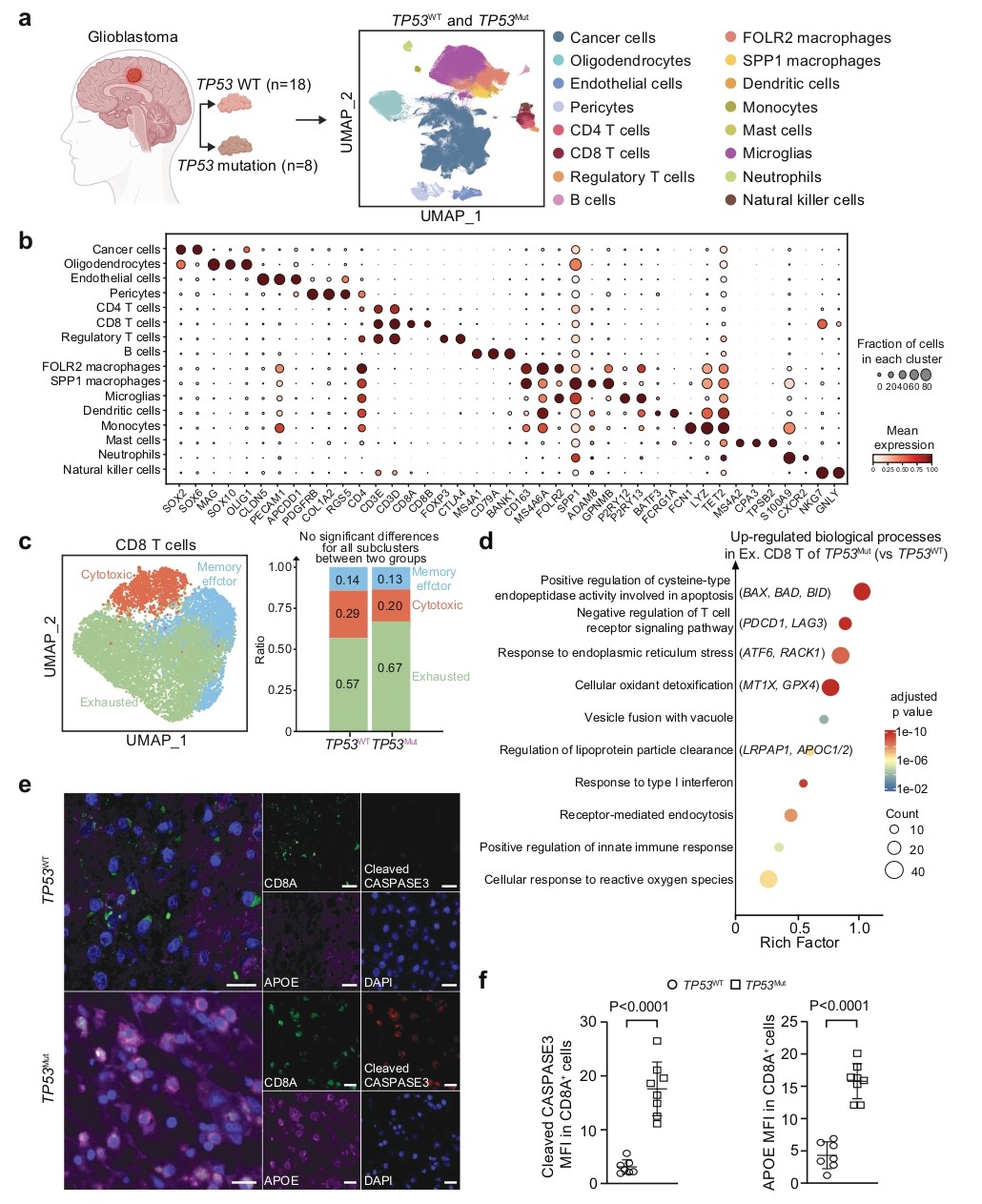
Figure 6. Infiltrating T Cells in TP53-mutated human GBM uptake APOE lipid particles and undergo apoptosis
Conclusion
This study is the first to fully elucidate a novel immune evasion pathway from "tumor necrosis" to "APOE release," leading to "lipid accumulation and functional exhaustion of immune cells," providing a new perspective for understanding tumor immune evasion mechanisms. The proposed anti-APOE therapeutic strategy, particularly in combination with immune checkpoint inhibitors, offers a new direction for immunotherapy of refractory tumors like glioblastoma. Given that APOE antibodies have already been under development for Alzheimer's disease, this strategy has promising clinical translation potential and is expected to bring new breakthroughs in cancer immunotherapy.
Although this study achieved positive results in mouse models, translating the anti-APOE antibody therapy to the clinic still faces challenges. For instance, optimizing the delivery efficiency of the antibody in humans and evaluating its long-term safety are required. Furthermore, how to precisely target necrotic tumor areas to maximize efficacy and minimize impact on normal tissues is an important direction for future research. Exploring the combined application of anti-APOE antibodies with other immunotherapeutic approaches (such as CAR-T cell therapy) also holds broad prospects.
