The 2025 Northern Hemisphere influenza season is characterized by a notably early onset. Surveillance data from both domestic and international sources indicate that the Influenza A(H3N2) virus has become the predominant circulating strain, presenting a challenging prevention and control situation.
Internationally, Japan was the first to enter its influenza epidemic season, approximately 5 weeks earlier than in 2024. During the week of October 6-12, over 9,000 influenza cases were reported from more than 3,800 sentinel medical institutions across the country, representing a 2.65-fold increase compared to the same period last year. Nearly a thousand schools suspended classes urgently due to outbreaks. The World Health Organization has indicated that the influenza epidemic in Japan is primarily driven by the Influenza A(H3N2) strain, which carries a risk of cross-regional transmission and could potentially spread to neighboring and other countries through travel.

Figure 1. Japanese Influenza Season News
Many European countries are also showing signs of increasing influenza activity. Significant recent rises in influenza cases in the UK, Germany, Italy, and other countries suggest that the Northern Hemisphere may face a prolonged winter influenza epidemic with a large scale of infections.
H3N2 Virus Introduction
H3N2 is an Influenza A virus that has been circulating continuously in humans since its first appearance in 1968, also known as Influenza A virus (IAV). H3N2 belongs to the family Orthomyxoviridae and is a single-stranded, negative-sense, segmented RNA virus. Its genome consists of eight negative-sense RNA segments, encoding 10 viral proteins: Polymerase Basic protein 2 (PB2), Polymerase Basic protein 1 (PB1), Polymerase Acidic protein (PA), Hemagglutinin (HA), Nucleoprotein (NP), Neuraminidase (NA), Matrix (M) protein, and the Nonstructural proteins NS1 and NS2.
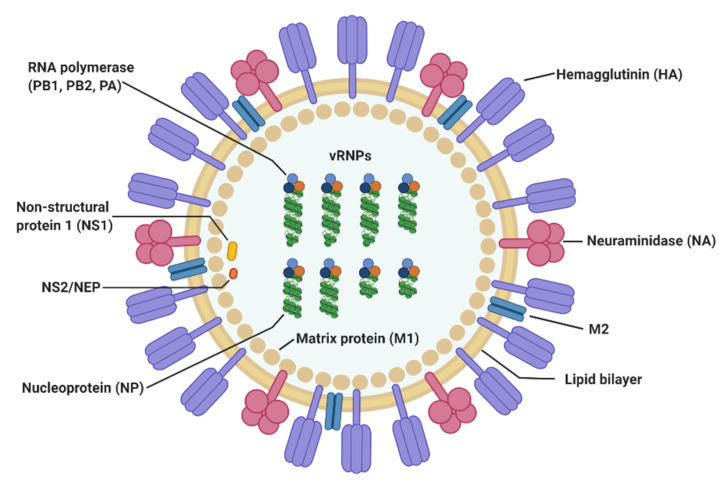
Figure 2. The structure of influenza A virus(Viruses. 2020 May 3;12(5):504.)
Table 1. Influenza Virus Protein Functions
|
Protein |
Function |
|
HA |
Envelope glycoprotein responsible for recognizing sialic acid receptors on host cell surfaces and mediating fusion of the viral envelope with the cell membrane. |
|
NA |
Envelope enzyme that cleaves sialic acid from host cell surfaces, aiding the release of newly formed virus particles and preventing aggregation. |
|
M2 |
Small transmembrane ion channel involved in the acidification process post-uncoating, crucial for viral replication; also a target for antiviral drugs (e.g., Adamantanes). |
|
NP |
Encapsulates viral RNA, forming ribonucleoprotein complexes, and participates in viral genome replication and transcription. |
|
PB2, PB1, PA |
Form the RNA-dependent RNA polymerase complex responsible for viral RNA replication and transcription. Mutations such as 627K/D701N in PB2 are closely associated with mammalian adaptation. |
|
NS1 |
Inhibits the host interferon response, aiding viral evasion of immune surveillance. |
After successful entry into a host cell, the influenza virus ultimately delivers its eight viral genome segments into the nucleus, initiating viral transcription and replication programs. The entire entry process can be divided into several key stages: First, the virion must recognize specific receptor molecules on the host cell surface, a mechanism ensuring the virus enters target cells suitable for its replication. The receptor recognized by influenza viruses is terminal α-sialic acid, linked via various forms to glycan chains anchored on the cell surface. Subsequently, the tight binding of the virion to the host cell activates endocytosis, forming an endosomal vesicle that encloses the virus. The endosome is then transported to a perinuclear region. A critical fourth step is mediated by the viral surface glycoprotein hemagglutinin: the viral envelope fuses with the endosomal membrane. This process prompts the release of all eight RNA genome segments into the nucleus, thereby initiating viral gene transcription and subsequent replication.
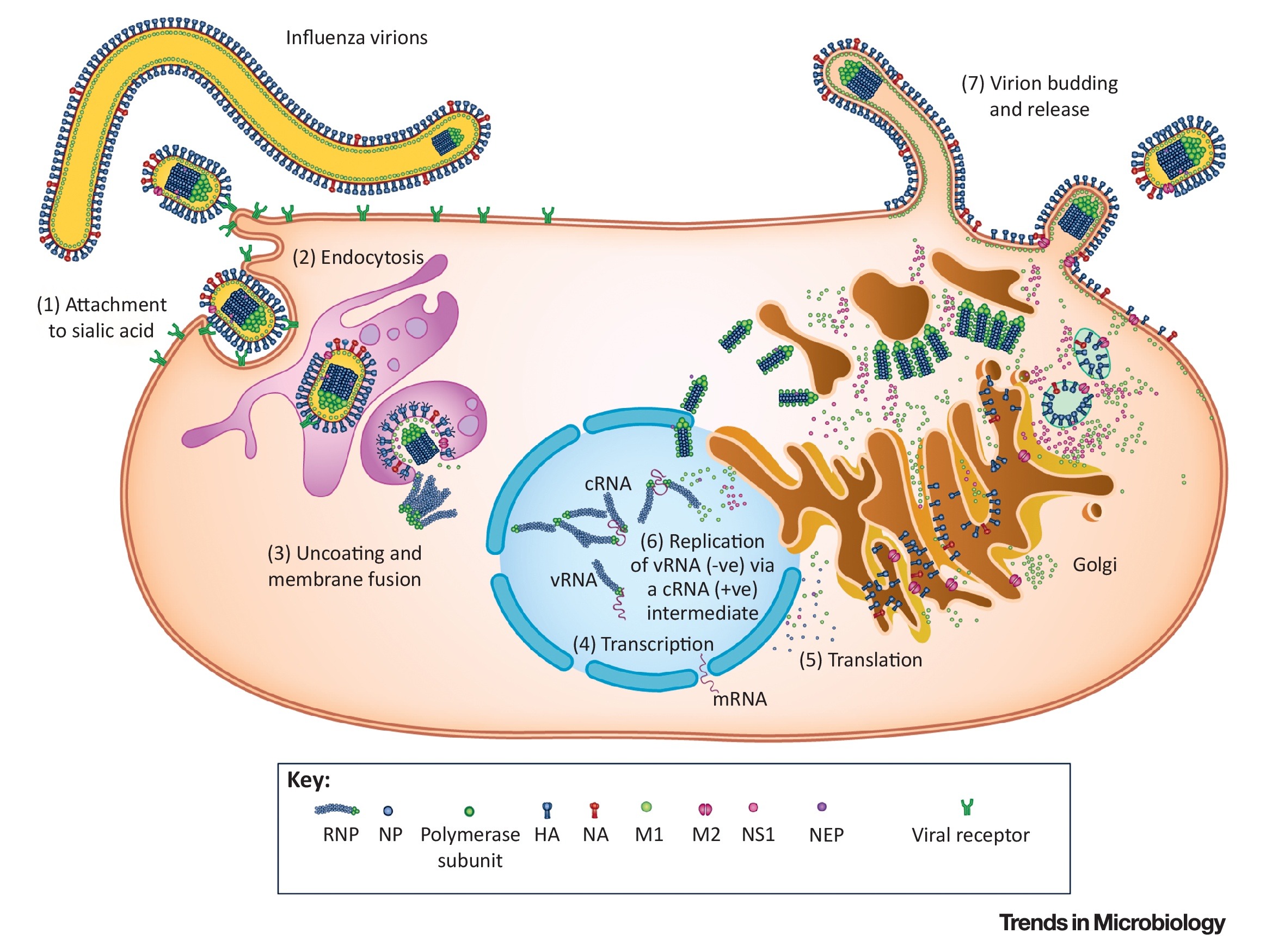
Figure 3. Replication cycle, and pathogenesis of influenza viruses(Trends Microbiol. 2018 Sep;26(9):809-810.)
Infection Prevention Measures
Vaccination remains the most effective means to prevent H3N2 infection and reduce the risk of severe illness and death. However, the H3N2 virus has a high frequency of antigenic drift, meaning its surface antigens (particularly the HA protein) are prone to mutation, allowing the virus to partially evade recognition by the host immune system. This characteristic makes H3N2 more likely to cause seasonal epidemics compared to H1N1 and poses a challenge to vaccine effectiveness!
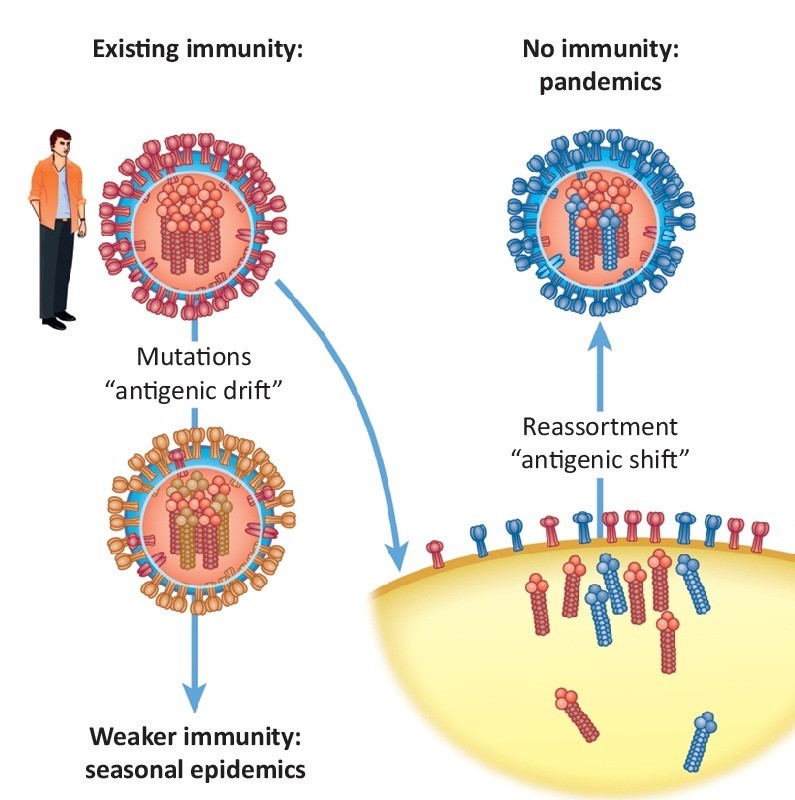
Figure 4. antigenic drift(Trends Microbiol. 2018 Sep;26(9):809-810.)
Currently, the primary influenza vaccines approved for global use are seasonal inactivated vaccines, which can be classified into trivalent and quadrivalent types based on the number of strains they cover. Trivalent inactivated vaccines protect against Influenza A(H1N1), A(H3N2), and Influenza B/Victoria lineage viruses. Quadrivalent inactivated vaccines add protection against the Influenza B/Yamagata lineage virus.
For the 2025 influenza season, with H3N2 as the dominant strain, quadrivalent vaccines offer relatively better protection due to their broader coverage. However, experts emphasize that if the quadrivalent vaccine is unavailable, receiving the trivalent vaccine is far superior to no vaccination and can significantly reduce the risk of severe outcomes. Additionally, a trivalent live attenuated vaccine is available for individuals aged 3-17 years; it induces an immune response by mimicking natural infection and demonstrates good immunogenicity.
However, seasonal influenza vaccines have notable limitations: first, the duration of protection is short, with vaccine-induced protective antibodies typically lasting only 6-8 months and waning over time; second, the match between the vaccine strain and circulating strains can vary significantly. Due to the rapid mutation rate of H3N2, mismatches frequently occur, leading to substantially reduced vaccine effectiveness.
Current Status of Influenza Vaccine Research
To overcome the limitations of seasonal vaccines, researchers are actively exploring novel vaccine technologies targeting H3N2, among which the universal influenza vaccine is one of the most promising directions. A research team at Georgia State University in the US used gene fusion technology to create a novel universal vaccine candidate by linking the highly conserved extracellular domain of the M2 protein (M2e) from H3N2 virus with the HA stalk protein. Animal studies showed that this vaccine induced specific antibodies against both M2e and the HA stalk in both adult and aged mice, while also activating protective T-cell immunity. It provided cross-protection against multiple Influenza A virus subtypes, including H3N2, H1N1, and H5N1. Furthermore, it can be produced on a large scale using bacterial cell culture, offering cost advantages.
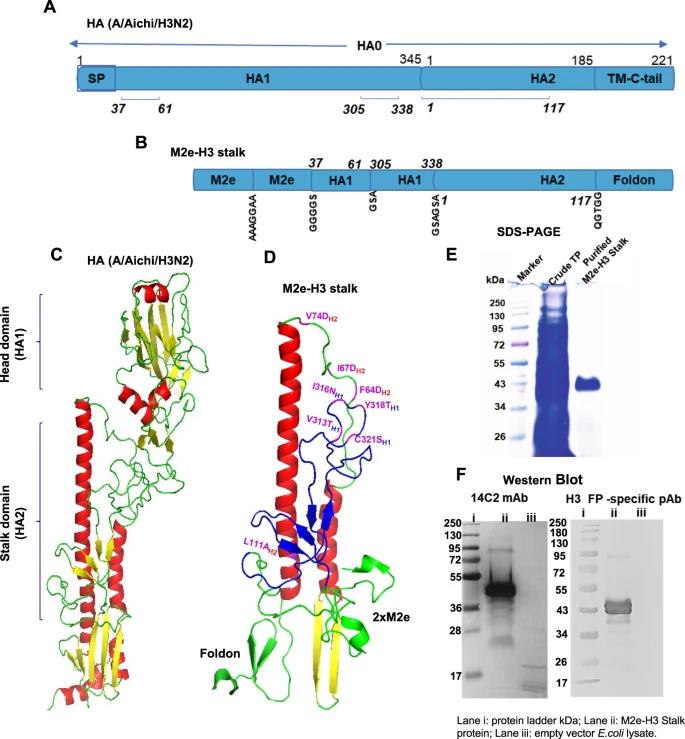
Figure 5. Rationale design of chimeric M2e-H3 stalk protein, purification, and confirmation(NPJ Vaccines. 2022 Jun 29;7(1):68.)
Additionally, H3N2 vaccines based on new technologies such as recombinant protein and mRNA are also under development. These technologies do not rely on egg-based cultivation, potentially significantly shortening the vaccine production cycle (from the traditional 6-8 months down to 1-2 months). This allows for a faster response to H3N2 variations and outbreaks, providing more efficient tools for pandemic influenza preparedness.
In summary, with its characteristics of rapid mutation and high pathogenicity, the Influenza A (H3N2) virus has become the central target for global influenza prevention and control in 2025. Although existing seasonal vaccines provide baseline protection, the development and application of novel universal vaccines will be key to ultimately overcoming the threat posed by H3N2. As the influenza season approaches, it remains crucial to get vaccinated promptly with available influenza vaccines and practice personal protective measures (such as wearing masks, frequent handwashing, and avoiding crowded places) to defend against H3N2 or other influenza virus strains.
References
1. Arevalo CP, Bolton MJ, Le Sage V, et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science. 2022;378(6622):899-904.
2. Subbiah J, Oh J, Kim KH, et al. A chimeric thermostable M2e and H3 stalk-based universal influenza A virus vaccine. npj Vaccines. 2022;7(1).
3. Hutchinson EC. Influenza Virus. Trends in Microbiology. 2018;26(9):809-810. doi:https://doi.org/10.1016/j.tim.2018.05.013
4. Luo M. Influenza virus entry. Advances in experimental medicine and biology. 2012;726:201-221.
5. Jung HE, Lee HK. Host Protective Immune Responses against Influenza A Virus Infection. Viruses. 2020;12(5):504.
AntibodySystem provides Influenza-related products, delivering more tools and solutions for research.
Recombinant Proteins
|
Catalog |
Product Name |
|
YVV23412 |
Recombinant Influenza A virus (H3N2) NR (N)-3M2e Protein, N-His |
|
YVV03302 |
Recombinant Influenza A virus (H3N2) NP/Nucleoprotein Protein, N-GST & C-His |
|
EVV03301 |
Recombinant Influenza A virus (H3N2) NP/Nucleoprotein Protein, C-His |
|
YVV23408 |
Recombinant Influenza A virus (H3N2) NF (CsgA)-3M2e Protein, N-His |
|
YVV24007 |
Recombinant Influenza A virus (H3N2) NA/Neuraminidase Protein, N-His |
|
EVV24002 |
Recombinant Influenza A virus (H3N2) NA/Neuraminidase Protein, N-His |
|
YVV23403 |
Recombinant Influenza A virus (H3N2) M2e Protein, N-GST & C-His |
|
YVV03811 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, N-His |
|
EVV03837 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-His |
|
EVV03832 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-His |
|
EVV03831 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-His |
|
EVV03830 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-His |
|
EVV03806 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-His |
|
EVV03804 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-His |
|
AVV03801 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-His (Active) |
|
EVV03833 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-8His |
|
EVV03829 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-8His |
|
EVV03836 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-6His |
|
EVV03834 |
Recombinant Influenza A virus (H3N2) HA/Hemagglutinin Protein, C-6His |
|
EVV03841 |
Recombinant Flu A/H3N2 HA/Hemagglutinin Protein, C-His |
|
EVV03840 |
Recombinant Flu A/H3N2 HA/Hemagglutinin Protein, C-His |
Antibodies
|
Catalog |
Product Name |
|
VVV24006 |
InVivoMAb Anti-Influenza A/B virus NA/Neuraminidase Broad-Neutralizing Antibody (Iv0283) |
|
VVV24001 |
InVivoMAb Anti-Influenza A virus Neuraminidase/NA Antibody (1G01) |
|
VVV03805 |
InVivoMAb Anti-Influenza A virus HA/Hemagglutinin Antibody (Iv0150) |
|
VVV03803 |
InVivoMAb Anti-Influenza A virus HA/Hemagglutinin Antibody (Iv0148) |
|
VVV03807 |
InVivoMAb Anti-Influenza A virus (H3N2) HA/Hemagglutinin Broadly Neutralizing Antibody (F045-092) |
|
PVV03302 |
Anti-Influenza A virus NP/Nucleoprotein Polyclonal Antibody |
|
RVV03302 |
Anti-Influenza A virus NP/Nucleoprotein Antibody (SAA0411) |
|
RVV24004 |
Anti-Influenza A virus Neuraminidase/NA Antibody (Mem5) |
|
RVV24006 |
Anti-Influenza A virus Neuraminidase/NA Antibody (B10) |
|
PVV24007 |
Anti-Influenza A virus (H3N2) NA/Neuraminidase Polyclonal Antibody |
|
PVV03811 |
Anti-Influenza A virus (H3N2) HA/Hemagglutinin Polyclonal Antibody |
|
DVV03807 |
Research Grade Anti-Influenza A virus HA/Hemagglutinin Broad-Neutralizing Antibody (CR9114) |
|
DVV03809 |
Research Grade Anti-Flu A (H3N2) HA/Hemagglutinin Broadly Neutralizing Antibody (CR8020) |
