In the field of immunology, immunoglobulin G (IgG) is widely regarded as the workhorse of humoral immunity. As the most abundant antibody in mammalian serum, it plays a critical role in defending against pathogenic infections.
This raises an intriguing question when we turn our attention to birds, such as chickens, which are closely associated with humans: Does the avian immune system also possess a functionally analogous—or even similarly named—molecule like IgG that serves as a key player in adaptive immunity?
In fact, chickens do produce a core antibody functionally equivalent to mammalian IgG. However, due to significant structural differences, it is scientifically classified as immunoglobulin Y (IgY).
The definitive answer is no; unlike mammals, birds do not express typical IgG. Instead, they rely on IgY as the primary antibody in serum and humoral immune responses. Due to their functional similarities, IgY was historically referred to as “chicken IgG“ before its distinct structural identity was fully characterized and the name IgY was standardized. In addition to IgY, IgM and IgA have been identified in avian species, while IgD and IgE have not been observed.
Structural Differences Between IgY and IgG
The IgY molecule shares a similar fundamental structure with IgG, comprising two heavy chains (H, ~67-70 kDa each) and two light chains (L, ~25 kDa each). The light chains have one constant region (CL) and one variable region (VL), similar to IgG. The major difference between IgY and IgG is found at the heavy chains. IgG has three constant regions at the heavy chains (CH1-CH3), while IgY has four constant regions (CH1-CH4). A further constant domain, with the corresponding carbohydrate chains, gives IgY a higher molecular weight (180 kDa) in comparison to IgG (150 kDa).
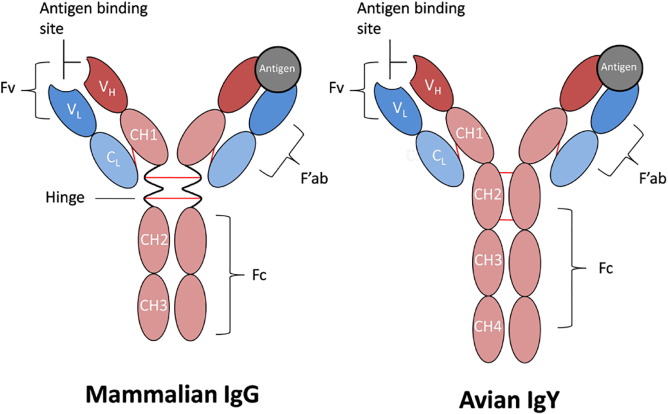
Fig. 1. The canonical structures of IgG and IgY(J Immunol Methods. 2017 Aug:447:71-85.)
IgY's Fc portion demonstrates significantly different glycosylation patterns and epitopes compared to mammalian IgG, preventing effective binding to mammalian Fcγ receptors (FcγR) or complement protein C1q. This characteristic enables IgY to function primarily as a pure antigen-binding fragment (Fab) in mammalian systems, with minimal initiation of effector functions such as antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) - a potential advantage in therapeutic applications.
Functionally, IgY plays a role in avian adaptive immunity parallel to that of IgG in mammals. It serves as the primary mediator of secondary immune responses and immunological memory, responsible for pathogen neutralization, opsonization for phagocytosis, and providing long-term protection against systemic infections. A crucial biological feature is the selective transport of serum-derived IgY into the yolk through an active process mediated by vitellogenin receptors, analogous to mammalian LDL transport. This mechanism enables developing chicken embryos and newly hatched chicks to acquire high concentrations of maternal antibodies, providing critical passive immune protection.
This unique transport pathway has facilitated the development of well-established IgY antibody technology. Immunizing hens induces high-titer, high-affinity antigen-specific IgY antibodies that become abundantly concentrated in egg yolk. Purifying IgY from yolk has emerged as an efficient, animal welfare-friendly alternative for large-scale polyclonal antibody production. The advantages are substantial: a single hen can produce hundreds of eggs annually, with each egg yielding 100-200 mg of total IgY, significantly surpassing the output of traditional rabbit antiserum collection methods. The immunization process requires minimal antigen and eliminates the need for repeated bleeding procedures in mammals.
The Fc region of IgY exhibits a distinct glycosylation pattern and epitope landscape compared to that of mammalian IgG. Consequently, it fails to effectively bind to mammalian Fc gamma receptors (FcγR) or activate the classical complement pathway via C1q.
Due to its unique Fc characteristics, IgY antibodies present low immunogenicity risks and are unlikely to trigger inflammatory side effects mediated by Fc-FcγR interactions when administered to mammalian systems. Consequently, yolk-derived IgY antibodies demonstrate substantial application potential and research value in multiple fields, including in vitro diagnostics, pathogen neutralization, and as antibiotic alternatives for preventing and treating gastrointestinal infections (such as oral IgY preparations against rotavirus or pathogenic E. coli).
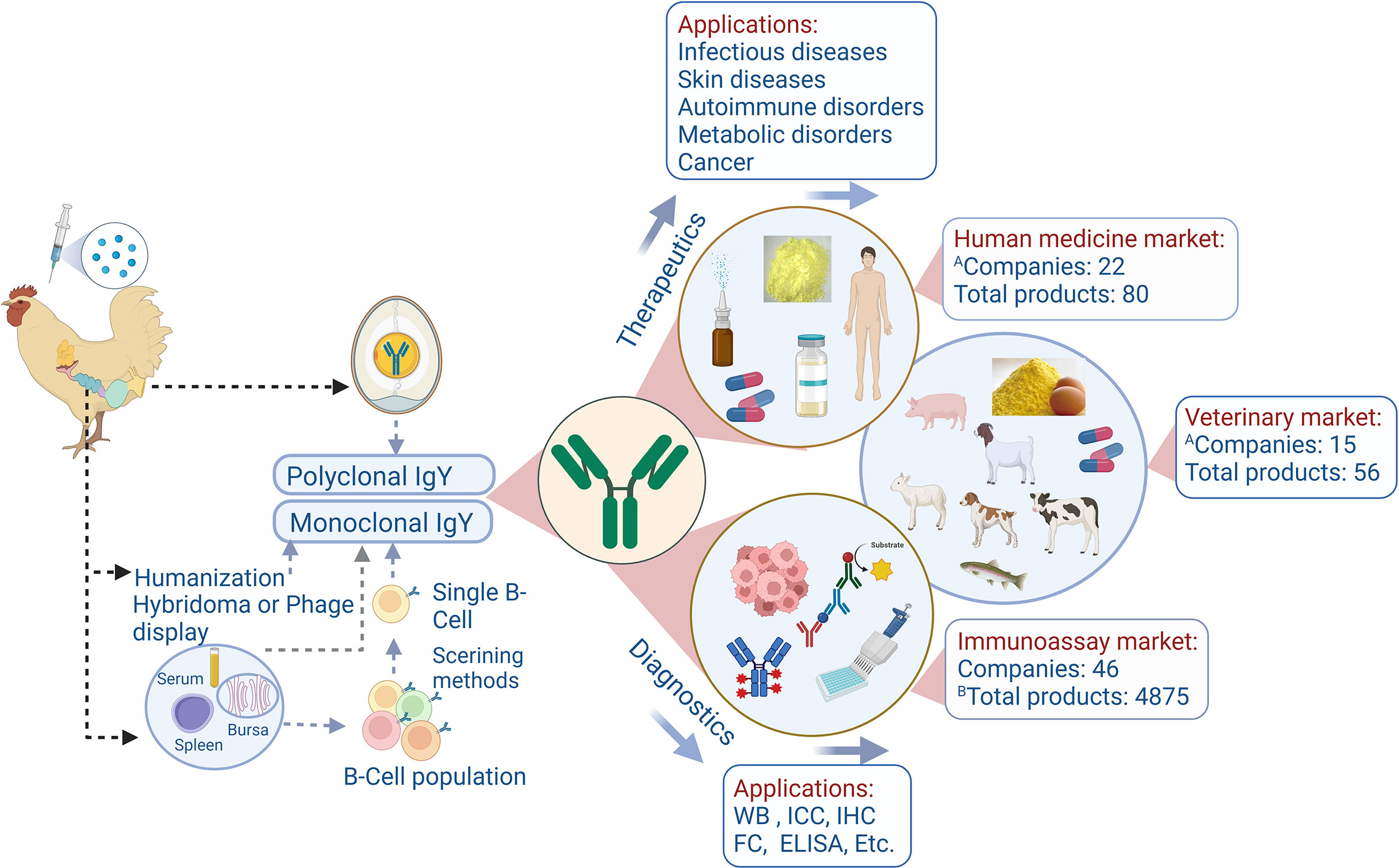
Fig. 2. Schematic indication of the IgY production, applications, and market status(Front Immunol. 2022 Oct 20;13:991931.)
Reference
1. Yakhkeshi S, Wu R, Chelliappan B, Zhang X. Trends in industrialization and commercialization of IgY technology. Frontiers in Immunology. 2022;13:991931.
2. Lee W, Syed Atif A, Tan SC, Leow CH. Insights into the chicken IgY with emphasis on the generation and applications of chicken recombinant monoclonal antibodies. Journal of Immunological Methods. 2017;447:71-85.
3. Zhang X, Calvert RA, Sutton BJ, Dor← KA. IgY: a key isotype in antibody evolution. Biological Reviews of the Cambridge Philosophical Society. 2017;92(4):2144-2156.
AntibodySystem provides IgY-related products, delivering more tools and solutions for related research.
|
Catalog |
Product Name |
|
ECC09201 |
Native Chicken IgY Protein |
|
PCC09211 |
Goat Anti-Chicken IgY Polyclonal Antibody |
|
PCC09231 |
Goat Anti-Chicken IgY (IgG) (H+L) Polyclonal Antibody, HRP |
|
FCC09210 |
Normal Chicken IgY Isotype Control Antibody |
|
FCC09211 |
Normal Chicken IgY Isotype Control Antibody, FITC |
|
FCC09212 |
Normal Chicken IgY Isotype Control Antibody, PE |
|
FCC09214 |
Normal Chicken IgY Isotype Control Antibody, PerCP |
|
FCC09213 |
Normal Chicken IgY Isotype Control Antibody, APC |
