Every September is World Alzheimer’s disease (AD) Month, AD is a neurodegenerative disorder characterized pathologically by the deposition of amyloid β-protein (Aβ) and hyperphosphorylation of tau protein. It clinically manifests as cognitive impairment, neuropsychiatric symptoms, and loss of social functioning.
AD as the most common neurodegenerative disorder, has become a major global public health challenge. According to statistics, approximately 55 million people worldwide are currently living with AD, and this number is projected to exceed 130 million by 2050, with over 60% of patients residing in developing countries. In terms of disease burden, global healthcare expenditures related to AD surpassed $1.3 trillion in 2023, and with an aging population, this cost continues to grow at an annual rate of 10%.
AD is a complex neurodegenerative disease influenced by a multitude of risk factors. These include genetic predisposition, the natural aging process, systemic inflammation, the presence of chronic diseases (type 2 diabetes, cardiovascular and cerebrovascular diseases), infections, traumatic brain injury (TBI), lifestyle choices (sleep patterns, high-fat, and high-salt diets), and environmental exposures. Additional factors that may affect AD incidence include neuropsychiatric symptoms, social engagement, alcohol consumption, hearing impairment, and educational attainment. The intricate interactions among these factors lead to the progressive neurodegeneration characteristic of AD.
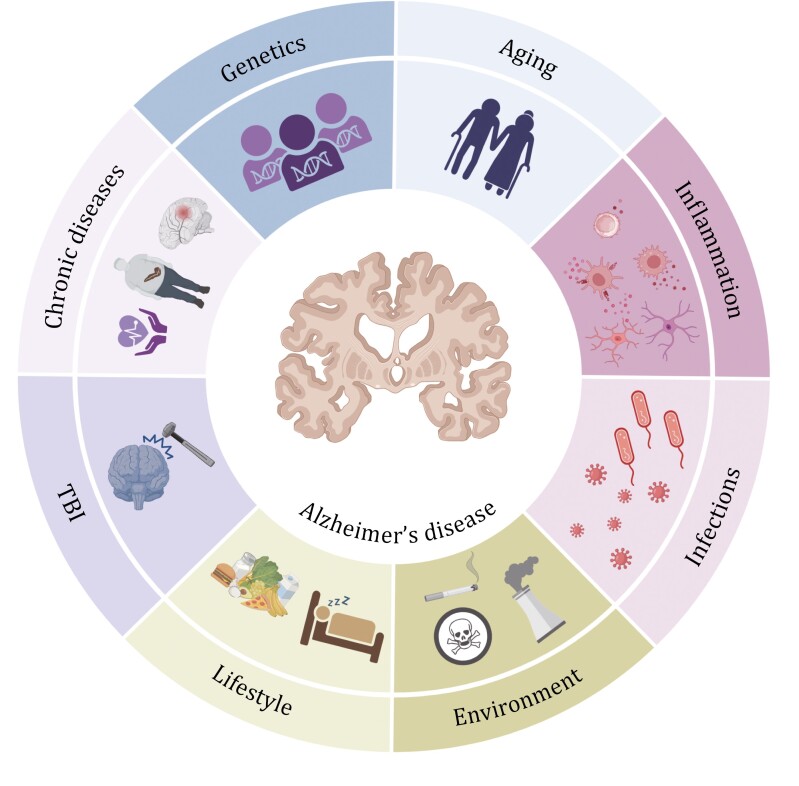
Fig.1 Diverse risk factors contributing to AD pathogenesis(Protein Cell. 2024 May 11;16(2):83–120.)
The core pathological features of AD include the deposition of amyloid-β (Aβ) plaques in the brain, neurofibrillary tangles formed by hyperphosphorylated tau protein, accompanied by neuronal death, synaptic loss, and neuroinflammatory responses. Although monoclonal antibodies targeting Aβ (such as Lecanemab and Donanemab) have received accelerated FDA approval in recent years, their clinical application carries risks such as intracranial hemorrhage and brain edema. Furthermore, they show limited efficacy only in early-stage AD patients and cannot reverse established neuronal damage. Concurrently, traditional symptomatic treatments (e.g., cholinesterase inhibitors, Memantine) can only alleviate symptoms but cannot halt disease progression. These clinical challenges underscore the urgent need to deeply understand AD pathological mechanisms and develop novel targeted therapies.
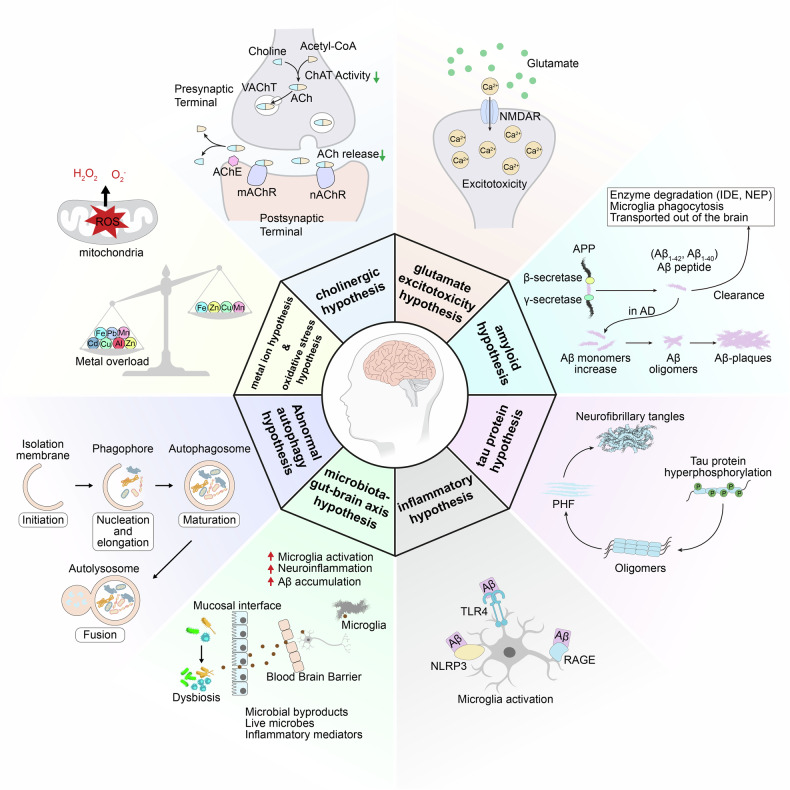
Fig.2 Diagram for the pathogenesis of AD(Signal Transduct Target Ther. 2024 Aug 23;9:211.)
In recent years, research focus has gradually shifted from singular "Aβ hypothesis" and "tau hypothesis" towards multi-target and multi-pathway synergistic regulatory mechanisms, such as glutamate toxicity, neuroinflammation, and pathology propagation mediated by extracellular vesicles. Based on several latest research findings, this article systematically elaborates on new discoveries in AD pathological mechanisms and potential therapeutic targets, providing a reference for research in the field.
New Discoveries in Alzheimer's Disease Pathogenesis and Therapeutic Targets
1. The NMDAR/TRPM4 Death Complex: A Key Mediator of Glutamate Toxicity in AD Progression
Research by Yan et al., published in Molecular Psychiatry under the title "The NMDAR/TRPM4 death complex is a major promoter of disease progression in the 5xFAD mouse model of Alzheimer's disease," discovered that the death signaling complex formed by extrasynaptic NMDAR and TRPM4 plays a central role in AD pathology. This complex is activated under conditions of disrupted glutamate homeostasis, inducing mitochondrial dysfunction, synaptic structural disintegration, and transcriptional dysregulation, thereby accelerating cognitive decline.
The study used oral administration of the small molecule inhibitor FP802 (a TwinF interface inhibitor) to specifically disrupt the NMDAR/TRPM4 complex without affecting the physiological functions of synaptic NMDARs. FP802 treatment significantly improved cognitive function in 5xFAD mice (assessed by Morris water maze, fear conditioning tests, etc.), preserved dendritic complexity, synaptic density, and mitochondrial structure, and even reduced Aβ plaque load. This suggests that targeting the NMDAR/TRPM4 complex may be a superior therapeutic strategy compared to traditional NMDAR blockers (like memantine), offering both neuroprotection and safety.
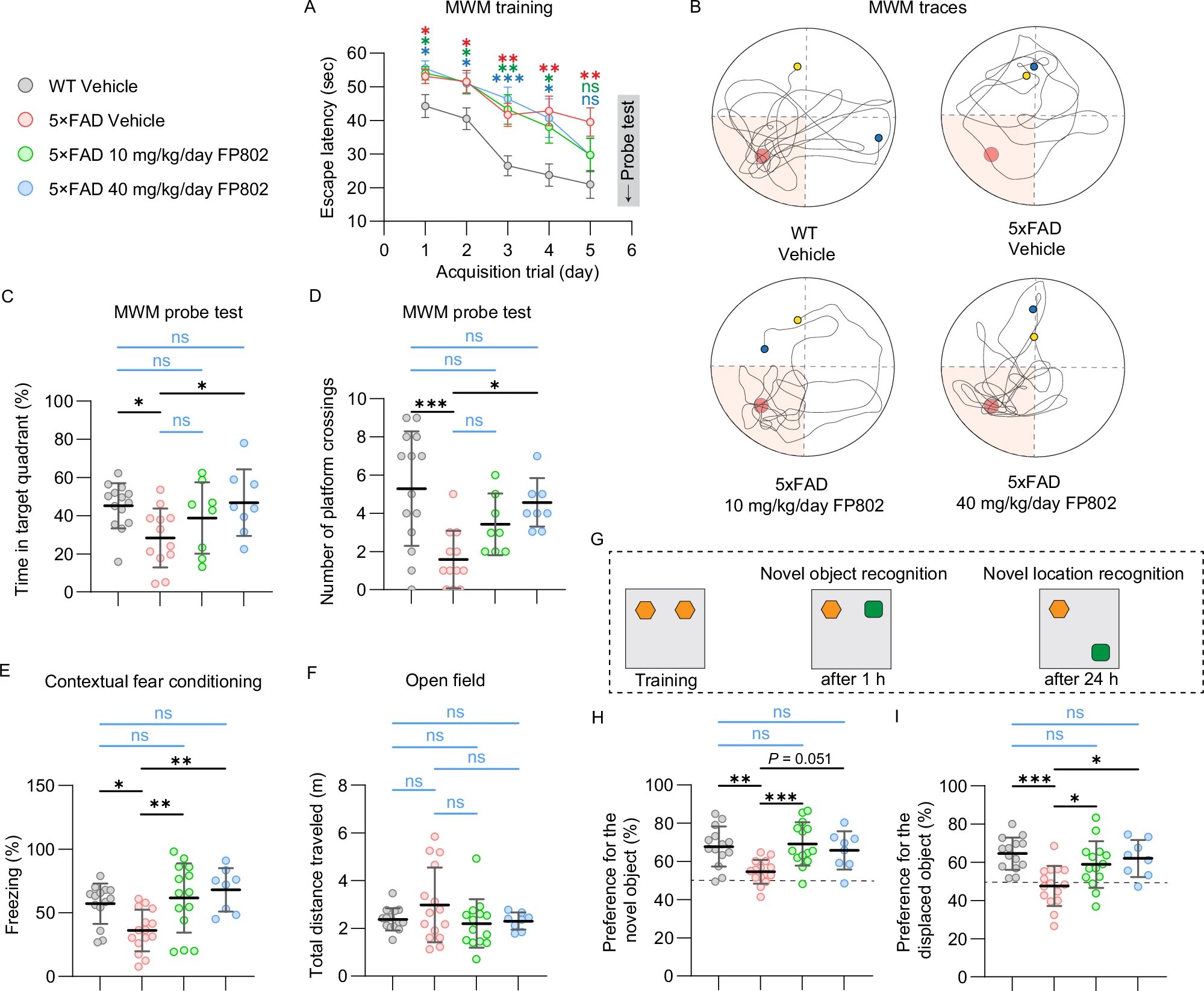
Fig.3 Effect of FP802 on cognitive function in 5xFAD mice(Mol Psychiatry. 2025 Aug 26.)
|
Catalog |
Product Name |
|
RHK33701 |
Anti-Human TRPM4/LTRPC4 Antibody (SAA0760) |
|
RHG32001 |
Anti-GluN2A/NR2A glutamate receptor Antibody (N327/95) |
|
RHG44801 |
Anti-GluN2B/NR2B glutamate receptor Antibody (N59/20) |
|
PHF37501 |
Anti-ACTB/β-actin/Beta Actin Polyclonal Antibody |
|
RHC12520 |
Anti-β-Amyloid (1-16aa) Antibody (6E10#) |
2. Peripherally-Derived EVs Carrying Complement C1q Promote Aβ Generation
Research by Yu et al., published in the Journal of Neuroinflammation under the title "Circulatory extracellular vesicles transport complement C1q for promoting neuronal amyloid-β production in Alzheimer's disease," revealed the role of extracellular vesicles (EVs) in the circulatory system during early AD pathology. They found that EVs isolated from the plasma of APP/PS1 mice (APPEVs) contained higher concentrations of the complement protein C1q compared to those from wild-type (WT) mice (WTEVs) and could cross the blood-brain barrier (BBB) without compromising its integrity.
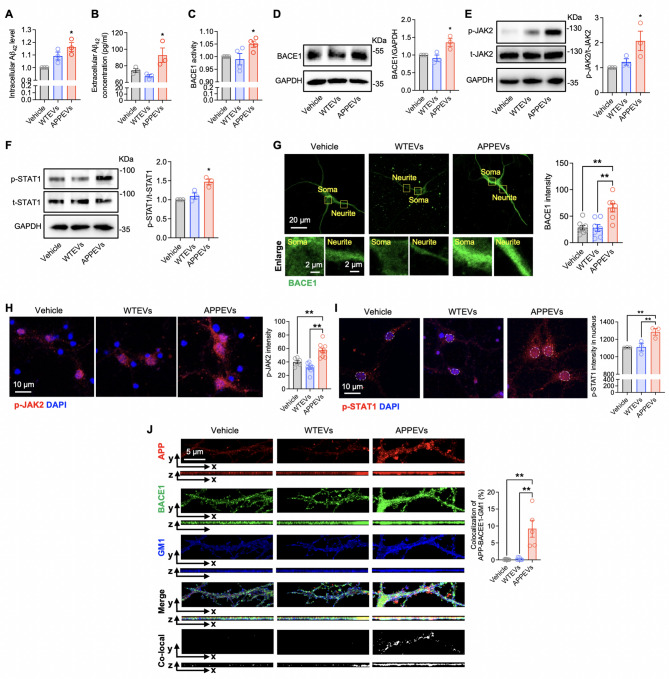
Fig.4 APPEVs induced neuronal Aβ42 production through JAK2-STAT1-BACE1 signaling(J Neuroinflammation. 2025 Aug 29;22(1):209.)
APPEVs activate the JAK2-STAT1 signaling pathway in neurons, upregulate the expression of β-secretase (BACE1), promote β-cleavage of APP within lipid rafts, and thereby increase Aβ42 generation. In vivo experiments showed that intravenous injection of APPEVs significantly enhanced Aβ plaque deposition in the brains of APP/PS1 mice, an effect that could be blocked by a C1q inhibitor. This research suggests that peripheral EVs may be important mediators connecting systemic inflammation with central Aβ pathology, offering new targets for early intervention.
|
Catalog |
Product Name |
|
RHC12520 |
Anti-β-Amyloid (1-16aa) Antibody (6E10#) |
|
RHF19603 |
Anti-BACE1 Antibody (R3N62) |
|
RHE34302 |
Anti-Phospho-STAT1 (S727) Antibody (R1V97) |
|
RHF19603 |
Anti-BACE1 Antibody (R3N62) |
|
RHC12517 |
Anti-APP Antibody (R2Y94) |
|
RHD16406 |
Anti-CD31/PECAM1 Antibody (R3E44) |
|
FHB76112 |
Anti-Human CLDN1/Claudin-1 Antibody (6F6C3), PE |
|
RHJ90501 |
Anti-Human CD321/F11R/JAM-A Antibody (SAA1942) |
3. CDK5-Mediated Tau217 Phosphorylation Exacerbates Synaptic Damage and Cognitive Impairment
Research by Fu et al., published in Translational Psychiatry under the title "CDK5-mediated hyperphosphorylation of Tau217 impairs neuronal synaptic structure and exacerbates cognitive impairment in Alzheimer's disease," focused on phosphorylation of tau protein at threonine 217 (p-T217), which has recently been recognized as an important biomarker for AD. The study found that elevated p-T217 levels were associated with CDK5 overactivation in 5xFAD mice.
By overexpressing the phospho-mimetic mutant TauT217E in the hippocampal region, researchers observed significant cognitive dysfunction (Y-maze and water maze tests), downregulation of synaptic proteins (PSD95, Drebrin), disrupted microtubule structure, and damage to synaptic ultrastructure. Conversely, the non-phosphorylatable mutant TauT217A had a protective effect. Further experiments confirmed that the CDK5/p25 complex directly phosphorylates Tau217, and this process could be inhibited by the CDK5 inhibitor Roscovitine. Reduced p-T217 levels in p35 knockout mice also supported the key regulatory role of CDK5.
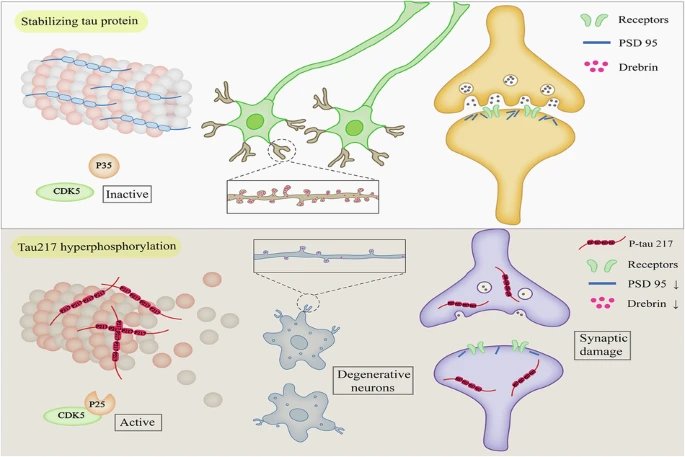
Fig.5 CDK5-mediated hyperphosphorylation of Tau217 impairs neuronal synaptic structure and exacerbates cognitive impairment in Alzheimer’s disease(Transl Psychiatry. 2025 Aug 21;15:302.)
|
Catalog |
Product Name |
|
PHF37501 |
Anti-ACTB/β-actin/Beta Actin Polyclonal Antibody |
|
RGK08501 |
Anti-Flag Tag (DYKDDDDK) Antibody (M2) |
|
RMJ92807 |
Anti-Mouse IgG1 Fc Antibody (SAA0489) |
|
RMC82401 |
Anti-Mouse MAPT/Tau/PHF-tau Antibody (TAU-5) |
|
PHF82201 |
Anti-CDK5 Polyclonal Antibody |
|
RHH32902 |
Anti-Drebrin 1/DBN1 Antibody (R3U45) |
|
PHF65301 |
Anti-Human DLG4/PSD95 Polyclonal Antibody |
|
PHF37501 |
Anti-ACTB/β-actin/Beta Actin Polyclonal Antibody |
|
PMB96411 |
Goat Anti-Mouse IgG H&L Polyclonal Antibody |
|
PTB96411 |
Goat Anti-Rabbit IgG H&L Polyclonal Antibody |
The above research expands our understanding of AD pathological mechanisms from different perspectives and provides diverse therapeutic targets. Mechanistically, these studies collectively reveal the "network nature" of AD pathology—Aβ, tau, glutamate toxicity, neuroinflammation, and peripheral signals do not act independently but form a pathological network through complex cross-regulations. This suggests that single-target interventions may be insufficient to effectively halt AD progression, and future strategies need to develop "multi-target synergistic" approaches.
From a therapeutic strategy perspective, these studies reveal several major trends: First, "precise targeting," such as FP802 targeting the NMDAR/TRPM4 complex interface and CDK5 inhibitors targeting Tau217 phosphorylation, avoids the non-specific side effects of traditional drugs. Second, "peripheral intervention," such as targeting C1q in circulating EVs, provides new pathways for drugs that cannot cross the blood-brain barrier.
AntibodySystem provides AD - related products, delivering more tools and solutions for brain health research.
APP/A beta, APOE, Tau, VLDLR, ACHE, TREM2, Nogo Receptor, BCHE, BACE-1, GSK-3beta, NPTX2, NEFL, TDP43, AD7c-NTP
References
1. Zheng Q, Wang X. Alzheimer’s disease: insights into pathology, molecular mechanisms, and therapy. Protein & Cell. 2024;16(2).
2. Zhang J, Zhang Y, Wang J, Xia Y, Zhang J, Chen L. Recent Advances in Alzheimer’s disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduction and Targeted Therapy. 2024;9(1).
3. Yan J, Yang X, Li G, et al. The NMDAR/TRPM4 death complex is a major promoter of disease progression in the 5xFAD mouse model of Alzheimer’s disease. Molecular psychiatry. Published online 2025:10.1038/s41380-02503143-5.
4. Fu K, Lin N, Xu Y, et al. CDK5-mediated hyperphosphorylation of Tau217 impairs neuronal synaptic structure and exacerbates cognitive impairment in Alzheimer’s disease. Translational Psychiatry. 2025;15(1).
