Aging is an inevitable process for every living organism and a current hotspot in life sciences research. From exploring the molecular mechanisms of aging to identifying interventions that delay aging, cellular senescence—as the cytological basis of organismal aging—has remained a major focus of the scientific community. Accurately defining cellular senescence and efficiently identifying senescent cells are crucial prerequisites for understanding the mechanisms of aging-related diseases and developing novel intervention strategies.
Cellular senescence is a stable and irreversible cell cycle arrest state that occurs in response to stress or damage (such as DNA damage, telomere attrition, oncogene activation, etc.), accompanied by a series of phenotypic and functional changes. Senescent cells no longer actively participate in tissue renewal; instead, they secrete a large number of cytokines, chemokines, proteases, and other factors, forming the Senescence-Associated Secretory Phenotype (SASP). While the SASP plays a physiologically protective role in embryonic development and tissue repair, its chronic accumulation can induce inflammatory responses and tissue functional decline, thereby driving the onset and progression of aging-related diseases such as neurodegenerative disorders, cardiovascular diseases, and cancer.
From a molecular mechanism perspective, the aging process is closely associated with 14 core hallmarks, including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled autophagy, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, dysbiosis, as well as the newly added extracellular matrix (ECM) changes and psychosocial isolation. These hallmarks are interconnected and collectively drive the aging process.
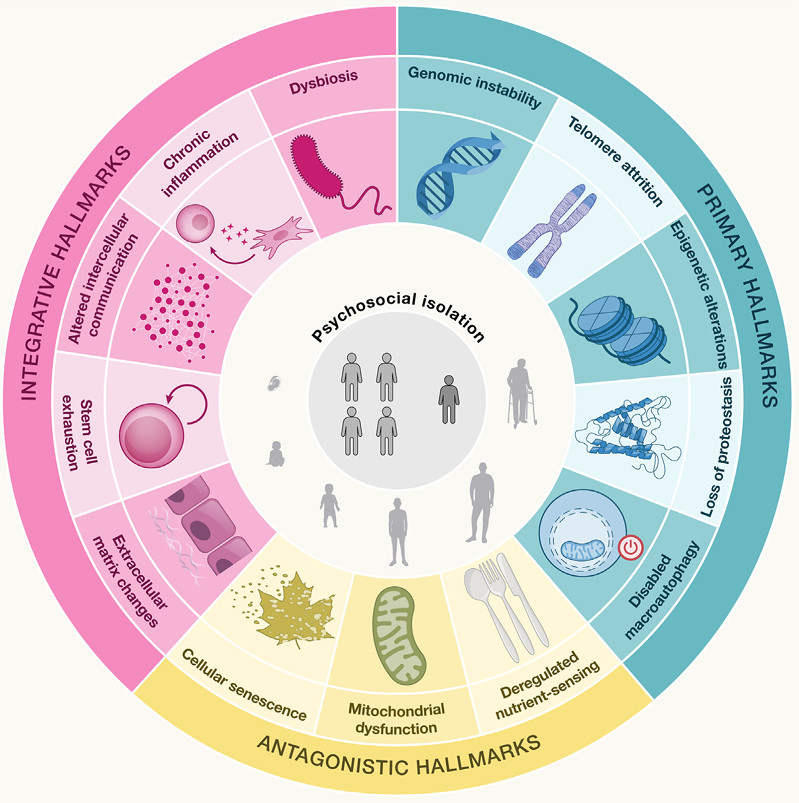
Fig 1. Schematic overview of the 14hallmarks of aging and their roles in the aging process at the primary, antagonist, and integrative levels(Cell. 2025 Apr 17;188(8):2043-2062.)
Accurate identification of senescent cells in complex tissues or in vitro models relies on a systematic understanding and multi-dimensional validation of their specific markers. Since a single marker is often insufficient to distinguish senescent cells from other dysfunctional cell states, it is necessary to integrate multiple categories of features for comprehensive assessment, including cell cycle arrest, DNA damage response (DDR), SASP, and altered lysosomal function.
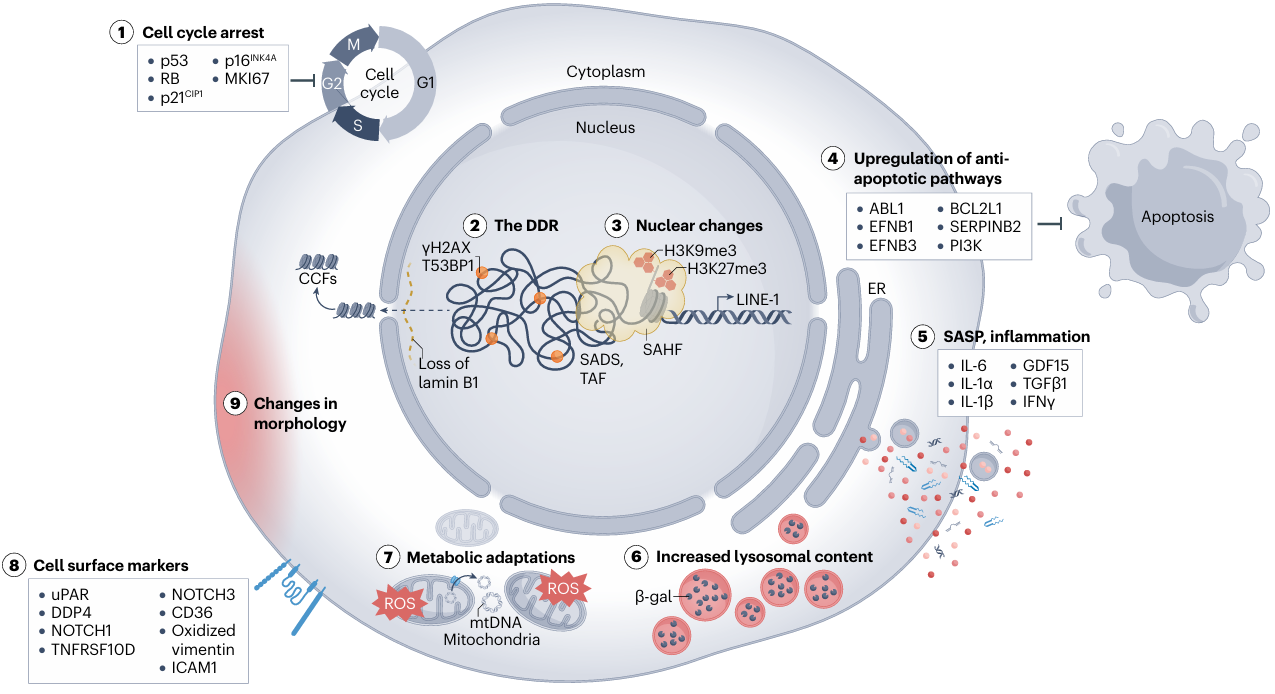
Fig 2. The hallmarks of cellular senescence(Nat Rev Mol Cell Biol. 2024 Dec;25(12):1001-1023.)
Among cell cycle arrest-related markers, CDKN2A (encoding p16INK4A) and CDKN1A (encoding p21CIP1) are the most classic core molecules. CDKN2A inhibits CDK4/6 kinase activity, arresting the cell cycle in the G1 phase. Its expression level significantly increases with age in multiple tissues and is specifically enriched in senescent cells. CDKN1A, regulated by p53, is rapidly upregulated in early senescence induced by DNA damage and serves as an important indicator of stress-induced senescence.
DNA damage response (DDR) markers are key clues revealing the triggers of cellular senescence. Among them, phosphorylated histone H2AX (γH2AX) forms foci at sites of DNA double-strand breaks and is a classical indicator for detecting DNA damage. Persistent accumulation of γH2AX foci can be observed in senescent cells. Additionally, TP53BP1, a key adaptor protein in the DDR pathway, often shows increased nuclear foci concomitant with accumulated DNA damage in senescent cells.
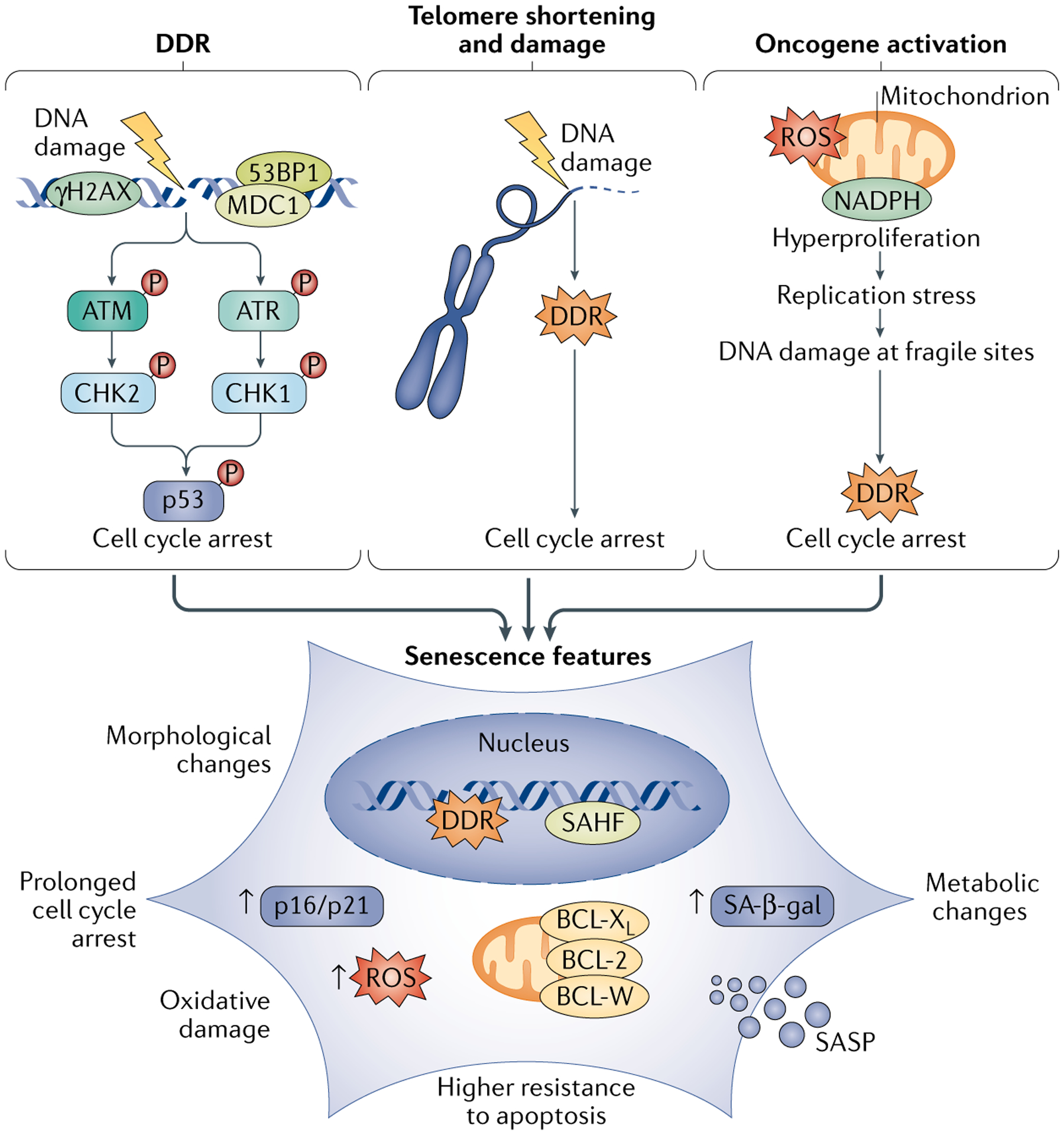
Fig 3. enescence drivers and phenotypes(Nat Rev Mol Cell Biol. 2021 Feb;22(2):75-95.)
SASP markers reflect the regulatory impact of senescent cells on the microenvironment. These markers exhibit significant cell-type and tissue specificity, though some core factors are highly conserved across various senescence contexts. Members of the interleukin family (IL-6, IL-1α, IL-1β) are core inflammatory factors of the SASP. Furthermore, SERPINE1 (encoding PAI-1), a serine protease inhibitor, is highly expressed in aged kidney and liver tissues and participates in tissue fibrosis by regulating extracellular matrix remodeling. Chemokines CCL2 and CCL3 contribute to the formation of an inflammatory microenvironment in aged tissues by recruiting immune cells. Their levels can be detected via ELISA or multiplex protein assays, and elevation reflects cellular senescence and a chronic inflammatory state.
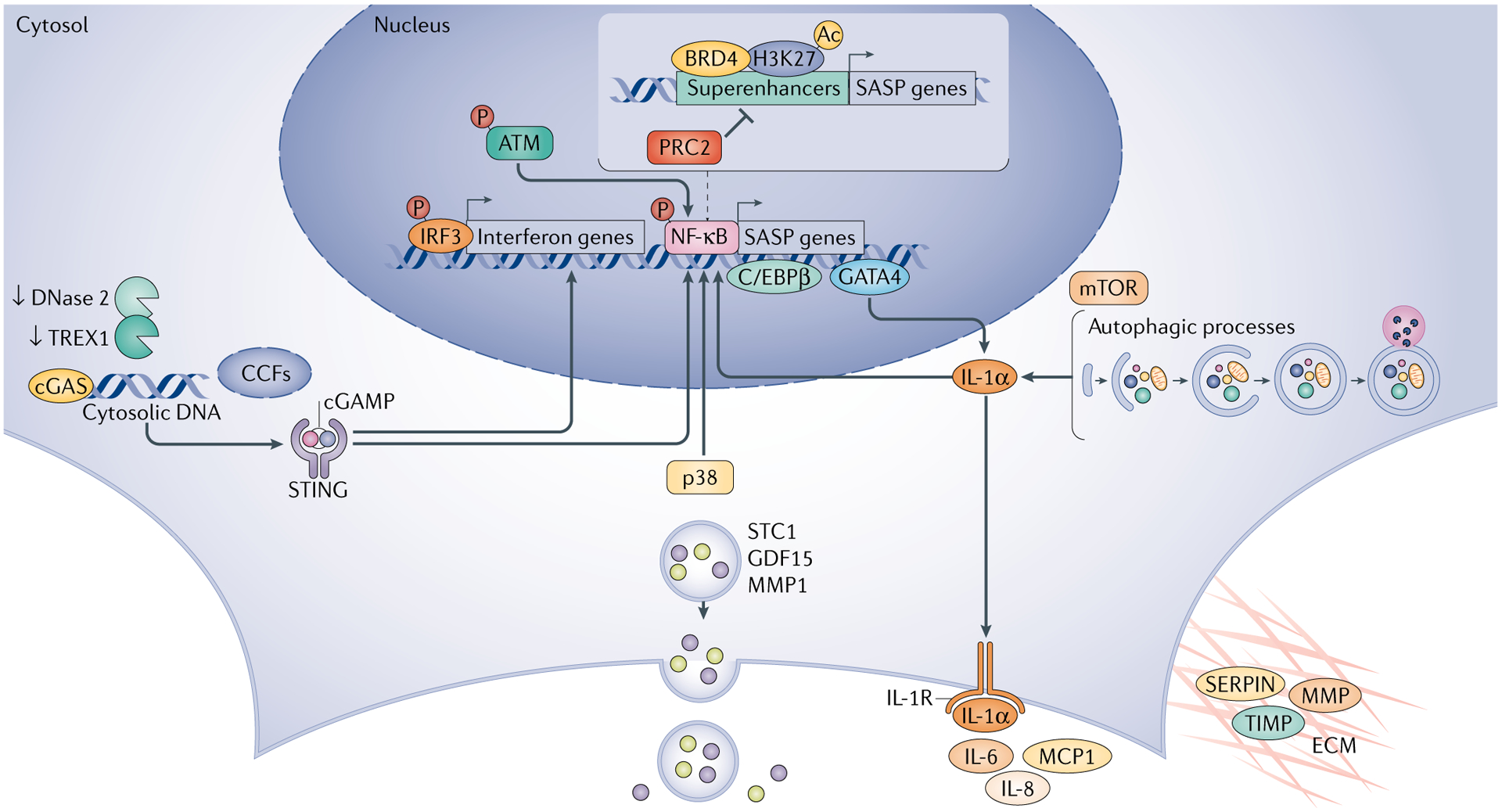
Among markers related to altered lysosomal function, senescence-associated β-galactosidase (SA-β-gal) is the most commonly used histological indicator. Due to increased lysosomal number and altered activity, senescent cells can be specifically stained by SA-β-gal at pH 6.0. This method is widely applied for detecting senescent cells in tissues such as skin, kidney, and pancreas.
Beyond the core categories mentioned above, markers related to nuclear structure and anti-apoptotic pathways also provide supplementary means for identifying senescent cells. Downregulation of the nuclear lamina protein LMNB1 leads to impaired nuclear membrane integrity, reported in senescent skin keratinocytes and neurons. Upregulation of the anti-apoptotic protein BCL2 and its family members (e.g., BCL2L1) is associated with the "anti-apoptotic phenotype" of senescent cells and can serve as potential targets for screening senolytics (drugs that clear senescent cells).
It is important to note that senescent cells in different tissues or disease models may present different combinations of markers. For instance, co-localization of CDKN2A and TAF is more prominent in in vitro models of replicative senescence, while expression of CDKN1A and γH2AX appears earlier in stress-induced senescence. Simultaneously, vigilance against "false-positive" signals is necessary. Therefore, accuracy in identifying senescent cells should be improved through methods such as co-localization of multiple markers and functional validation.
References
1. Kroemer G, Maier AB, Cuervo AM, et al. From geroscience to precision geromedicine: Understanding and managing aging. Cell. 2025;188(8):2043-2062.
2. López-Otín, C, Blasco, M. A, Partridge, L, et al. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186 (2), 243–278.
3. Suryadevara V, Hudgins AD, Rajesh A, et al. SenNet recommendations for detecting senescent cells in different tissues. Nature Reviews Molecular Cell Biology. 2024;25(12):1001-1023.
4. Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular Senescence in ageing: from Mechanisms to Therapeutic Opportunities. Nature Reviews Molecular Cell Biology. 2020;22(2):75-95.
AntibodySystem provides Cellular senescence -related products, delivering more tools and solutions for the mechanisms of aging-related diseases research.
|
Catalog |
Product Name |
|
PHD16301 |
Anti-Human GLB1/Beta-galactosidase Polyclonal Antibody |
|
RHD16302 |
Anti-GLB1 Antibody (R1Q04) |
|
FHC10011 |
Anti-Human TP53/p53 (R175H mutant) Antibody (F2), FITC |
|
RHC10008 |
Anti-TP53/p53 Antibody (R2Y81) |
|
PHE23501 |
Anti-p21/CDKN1A Polyclonal Antibody |
|
PHK35501 |
Anti-Human CDKN2A Polyclonal Antibody |
|
RHE37704 |
Anti-CDKN2A/p16INK4a Antibody (R3K92) |
|
RHE37705 |
Anti-CDKN2A/p16INK4a Antibody (R3K93) |
|
VMB95601 |
InVivoMAb Anti-Mouse/Rat IL-1β Antibody (B122) |
|
VHC97901 |
InVivoMAb Anti-Human CCL5/RANTES (Iv0079) |
|
PHC97901 |
Anti-CCL5 Polyclonal Antibody |
|
VHC01901 |
InVivoMAb Anti-Human CXCL10/IP-10 (Iv0088) |
|
VHC15801 |
InVivoMAb Anti-Human IL6 (Iv0022) |
|
RHC15803 |
Anti-Human IL6 Antibody (SAA2008) |
|
RHD15005 |
Anti-Human H2AX Nanobody (SAA1140) |
|
RHD15010 |
Anti-H2AX Antibody (R1P93) |
|
RHD40901 |
Anti-LMNB1 Antibody (R1A03) |
|
PHD40901 |
Anti-LMNB1 Polyclonal Antibody |
|
RHC81005 |
Anti-BCL2 Antibody (R3C07) |
|
PHG32401 |
Anti-TP53BP1 Polyclonal Antibody |
|
PHE46101 |
Anti-Ki67/MKI67 Polyclonal Antibody |
