Catalog No.
KAC09601
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Trastuzumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and Anti-Trastuzumab will be captured by immobilized Trastuzumab. After washing away any unbound substances, a biotin-labeled Trastuzumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Trastuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
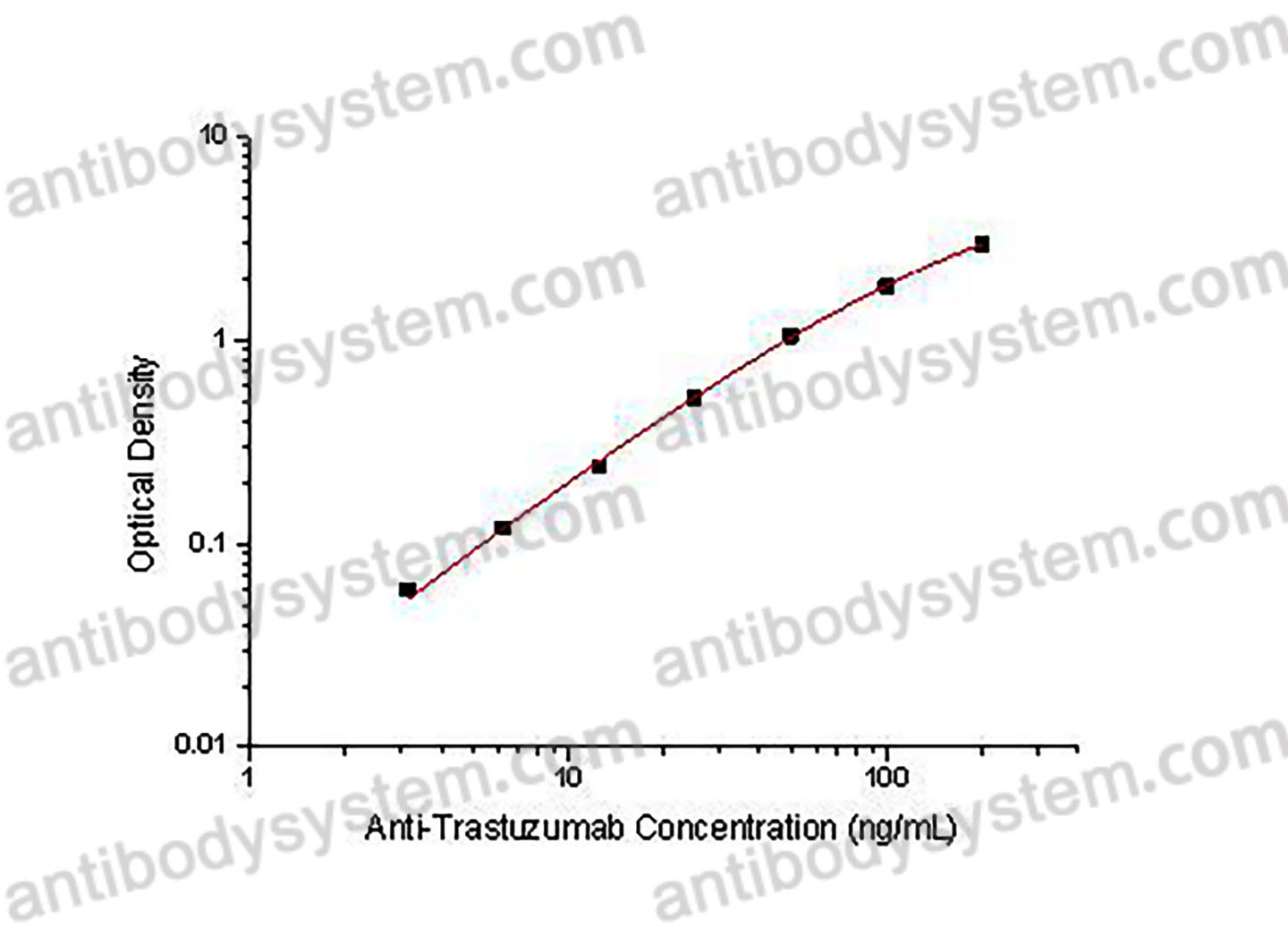
Range
3.13 - 200 ng/mL
Sensitivity
2.94 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
92.9
|
25.0
|
10.2
|
89.4
|
23.1
|
10.5
|
|
Standard deviation
|
4.2
|
1.4
|
0.8
|
8.8
|
1.9
|
0.9
|
|
CV (%)
|
4.5
|
5.6
|
7.9
|
9.9
|
8.3
|
8.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
timigutuzumab, 4D5-8, 4D5V8, Herceptin, rhuMab HER2,CAS: 180288-69-1
Medicare spending and use of subcutaneous biologic formulations with hyaluronidase., PMID:40515473
Association of HER2-low with clinicopathological features in patients with early invasive lobular breast cancer: an international multicentric study., PMID:40514709
Pharmacokinetic bioequivalence of the fixed-dose combination of pertuzumab and trastuzumab administered subcutaneously using a handheld syringe or an on-body delivery system., PMID:40514611
Drug-drug interaction between trastuzumab emtansine (T-DM1) and orally administered tacrolimus in a patient and in rats., PMID:40513507
ctDNA Analysis in ERBB2-Amplified Colorectal Cancer: Biomarker Analysis of the MyPathway Trial., PMID:40512191
Advancing Antibody-Drug Conjugates: Precision Oncology Approaches for Breast and Pancreatic Cancers., PMID:40507272
Preirradiation of Spheroids with 225Ac-Trastuzumab Improves Penetration of 225Ac-Liposomes and MIRDcell Predictions of Responses to Drug Cocktails., PMID:40506236
[Effect of taste perception on nutritional status in patients with breast cancer: a systematic review]., PMID:40504002
Plasma IFN-γ may predict pyrotinib efficacy in patients with HER2-positive advanced breast cancer., PMID:40502358
Comparative pharmacological analysis of fam-trastuzumab deruxtecan-nxki and sacituzumab govitecan-hziy: Two recently developed chemotherapies in the crucial battle against breast cancer., PMID:40502326
Reply to Letter to the Editor: 'A pooled analysis of trastuzumab deruxtecan in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer with brain metastases' by Y. Liu & C. Zhang., PMID:40500685
Strong Hsp90α/β Protein Expression in Advanced Primary CRC Indicates Short Survival and Predicts Response to the Hsp90α/β-Specific Inhibitor Pimitespib., PMID:40498011
Beyond the Tumor: Invasive Fungal Infection Unveiled in HER2-Positive Breast Cancer Patient Mimicking Disease Relapse., PMID:40496029
Analytical and clinical validation of PATHWAY HER2 (4B5) assay for assessment of HER2-low/HER2-ultralow status and eligibility for trastuzumab deruxtecan in DESTINY-Breast06., PMID:40494041
Long and short-term efficacy and safety comparison of nab-paclitaxel versus paclitaxel combined with trastuzumab and pertuzumab for neoadjuvant treatment of HER2-positive breast cancer: A systematic review and meta-analysis., PMID:40493995
Advanced 64Cu/67Cu-Based Theranostics: Application of the Copper Chelator TE1PA to a Murine Model of HER2-Positive Gastric Cancer., PMID:40493435
Effects of concurrent HER2-directed therapy on development of cerebral radionecrosis after stereotactic radiotherapy: a systematic review., PMID:40493045
Novel Quaternary Ammonium Salt-Linked STING Agonist Antibody-Drug Conjugate: Synergistic Activation of Tumor Immunity with Mitigated Off-Target Toxicity., PMID:40492586
Reinforced Polymer-Nanoparticle Hydrogels for Subcutaneous and Sustained Delivery of Trastuzumab., PMID:40489148
A Randomized Phase 2 Study of Neratinib With or Without Fulvestrant for Patients With HER2-Positive, Estrogen Receptor-Positive Metastatic Breast Cancer., PMID:40484776
Treatment of breast cancer in a patient with sickle cell disease., PMID:40484435
Real-World 5-Year Outcomes of Patients Treated With the Adjuvant Paclitaxel Trastuzumab (APT) Regimen for Stage I HER2-Positive Early Breast Cancer: A Retrospective International Study., PMID:40483197
Advancing Neoadjuvant Therapy with Inetetamab for HER2-Positive Breast Cancer., PMID:40482910
Proteogenomic analysis of the CALGB 40601 (Alliance) HER2+ breast cancer neoadjuvant trial reveals resistance biomarkers., PMID:40480221
Spatial discovery of pyrotinib overcoming HER2-positive breast cancer resistance by breaking fibroblast-induced immune barriers., PMID:40480079
The role of HER2-targeted therapy in urothelial carcinomas., PMID:40478157
Site-Specific Immune and Stromal Architecture Drive Resistance to Trastuzumab Deruxtecan in HER2+ Metastatic Breast Cancer., PMID:40475468
Design, Synthesis, and Biological Evaluation of Evodiamine Derivatives as Antibody-Drug Conjugate (ADC) Payloads., PMID:40474320
225Ac α-Pretargeted Radioimmunotherapy of Human Epidermal Growth Factor Receptor 2-Expressing Breast Cancer., PMID:40473462
Quantitative [89Zr]Zr-Trastuzumab PET and Diffusion- Weighted MRI for Characterization of Metastatic HER2-Positive Breast Cancer with PET/MRI., PMID:40473459
Maintenance with niraparib in patients with stage III, stage IV, chemo-naïve recurrent or platinum-sensitive recurrent uterine serous carcinoma: study protocol for a phase II clinical trial., PMID:40473279
Advances and Future Perspectives of HER2 Mutations in Non-Small Lung Cancer (NSCLC), Especially in China., PMID:40472040
Trastuzumab-deruxtecan shows interesting disease control in a multitreated patient with ERBB2 amplified parotid carcinoma, a case report., PMID:40470107
Trastuzumab monotherapy as maintenance treatment for metastatic HER2+ vulvar Paget disease: systematic review and case report., PMID:40469473
TCHL - a phase II neo-adjuvant study assessing TCH (docetaxel, carboplatin and trastuzumab) and TCHL (docetaxel, carboplatin, trastuzumab and lapatinib) in HER-2 positive breast cancer patients: a 5-year follow-up with serum biomarker analysis., PMID:40468999
Successful Treatment of HER2 V659E Mutation-Positive Lung Adenocarcinoma With Trastuzumab Deruxtecan: A Case Report., PMID:40468744
Continuous glucose monitoring to characterize hyperglycemia during chemotherapy for early stage breast cancer., PMID:40468140
Improving the Therapeutic Selectivity of Trastuzumab Deruxtecan Using 8C2 Fab Fragments., PMID:40467988
Adverse effect of trastuzumab deruxtecan in solid tumours: a systematic review and meta-analysis., PMID:40466817
Targeting SLC7A11-mediated cysteine metabolism for the treatment of trastuzumab-resistant HER2-positive breast cancer., PMID:40464376
The radioactive 103Pd and 109Pd palladium bipyridyl-bisphosphonate complexes for radionuclide therapy of bone metastatic tumor cells., PMID:40463346
Long-Term Safety and Efficacy of the Fixed-Dose Combination of Pertuzumab and Trastuzumab for Subcutaneous Injection in Patients With HER2-Positive Early Breast Cancer in PHranceSCa, a Randomized, Open-Label Phase II Study., PMID:40461387
Mechanisms of resistance to antibody-drug conjugates in cancers., PMID:40460510
New Treatment Approaches for Triple-Negative Breast Cancer., PMID:40460322
Superhydrophilic Multichannel TiO2 Fibers for Simultaneous Enrichment of O-GalNAc Glycopeptides and Phosphopeptides., PMID:40460305
Trastuzumab Deruxtecan or Ramucirumab plus Paclitaxel in Gastric Cancer., PMID:40454632
The Rapidly Evolving Landscape of Human Epidermal Growth Factor Receptor 2 (HER2) Testing in Endometrial Carcinoma and Other Gynecologic Malignancies., PMID:40452624
Radionuclide-Aided Improvement of the Therapeutic Efficacy of Novel Antibody-Drug Conjugates against HER2-Expressing Cancers., PMID:40448644
EXPRESS: Advanced Therapies for Stomach Cancer., PMID:40448345
Radiobioconjugate of Kadcyla with Radioactive Gold Nanoparticles for Targeted Therapy of HER2-Overexpressing Cancers., PMID:40443068