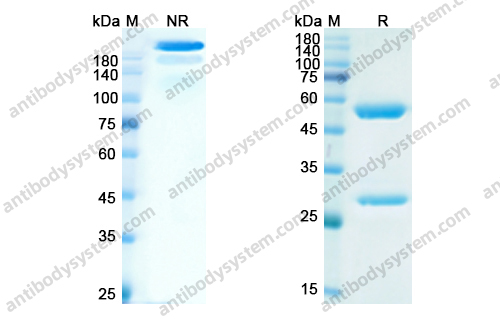
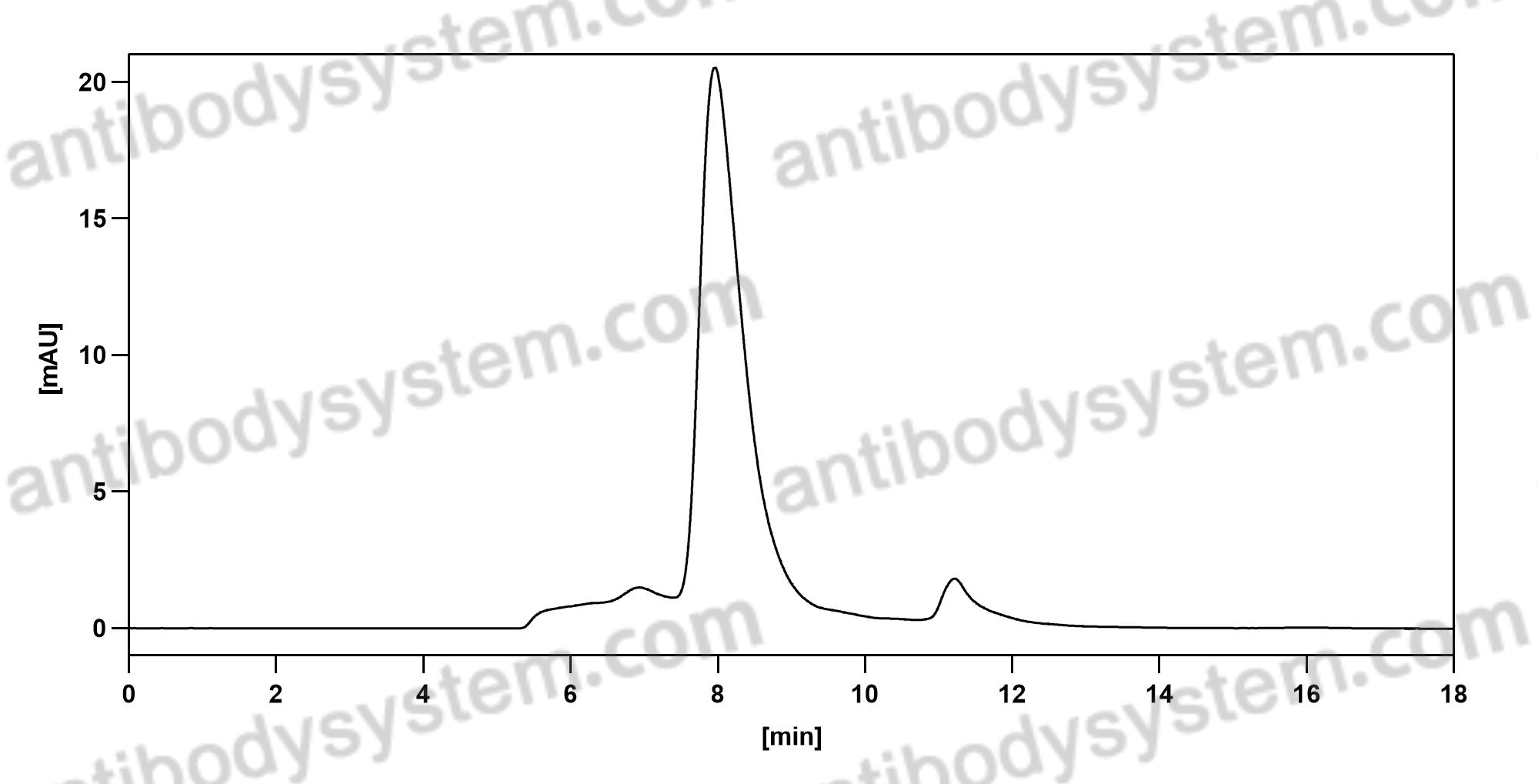
Catalog No.
DHC21901
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG2-kappa
Clonality
Monoclonal
Target
MSK8, VNRA, Vitronectin receptor, Integrin alpha-V, Vitronectin receptor subunit alpha, CD51, ITGAV, VTNR
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P06756
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
DI-17E6,EMD 525797, CAS: 1105038-73-0
Clone ID
Abituzumab
STRATUS: A Phase II Study of Abituzumab in Patients With Systemic Sclerosis-associated Interstitial Lung Disease, PMID: 33004536
Abituzumab Targeting of αV-Class Integrins Inhibits Prostate Cancer Progression, PMID: 28314844
Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial, PMID: 25319061
Differential Effect on Bone Lesions of Targeting Integrins: Randomized Phase II Trial of Abituzumab in Patients with Metastatic Castration-Resistant Prostate Cancer, PMID: 26839144
Integrins as A New Target for Cancer Treatment, PMID: 30451118
Integrin Inhibitors in Prostate Cancer, PMID: 29415418
Integrins as Therapeutic Targets: Successes and Cancers, PMID: 28832494
Ongoing clinical trials and treatment options for patients with systemic sclerosis-associated interstitial lung disease, PMID: 29893938
A novel therapeutic option for castration-resistant prostate cancer: after or before chemotherapy?, PMID: 23838638
Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles, PMID: 20031203
Safety, tolerability, and pharmacokinetics of the novel αv-integrin antibody EMD 525797 (DI17E6) in healthy subjects after ascending single intravenous doses, PMID: 24242902
A multicenter phase 1 study of EMD 525797 (DI17E6), a novel humanized monoclonal antibody targeting αv integrins, in progressive castration-resistant prostate cancer with bone metastases after chemotherapy, PMID: 23791392
Inside a Metastatic Fracture: Molecular Bases and New Potential Therapeutic Targets., PMID:40304052
Integration of NMR Spectroscopy in an Analytical Workflow to Evaluate the Effects of Oxidative Stress on Abituzumab: Beyond the Fingerprint of mAbs., PMID:37278511
STRATUS: A Phase II Study of Abituzumab in Patients With Systemic Sclerosis-associated Interstitial Lung Disease., PMID:33004536
Integrins as A New Target for Cancer Treatment., PMID:30451118
Ongoing clinical trials and treatment options for patients with systemic sclerosis-associated interstitial lung disease., PMID:29893938
Integrin Inhibitors in Prostate Cancer., PMID:29415418
Integrins as Therapeutic Targets: Successes and Cancers., PMID:28832494
Abituzumab Targeting of αV-Class Integrins Inhibits Prostate Cancer Progression., PMID:28314844
Differential Effect on Bone Lesions of Targeting Integrins: Randomized Phase II Trial of Abituzumab in Patients with Metastatic Castration-Resistant Prostate Cancer., PMID:26839144
Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial., PMID:25319061
Safety, tolerability, and pharmacokinetics of the novel αv-integrin antibody EMD 525797 (DI17E6) in healthy subjects after ascending single intravenous doses., PMID:24242902
A novel therapeutic option for castration-resistant prostate cancer: after or before chemotherapy?, PMID:23838638
A multicenter phase 1 study of EMD 525797 (DI17E6), a novel humanized monoclonal antibody targeting αv integrins, in progressive castration-resistant prostate cancer with bone metastases after chemotherapy., PMID:23791392
Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles., PMID:20031203