January 13th, 2026, a study published in Cell Stem Cell has drawn significant attention from the reproductive medicine and stem cell research communities. In the article entitled “Development of human induced pluripotent stem cell-derived ovarian support cells as a clinical-grade product for in vitro fertilization,” researchers from Gameto report the development of ovarian support cells (OSCs) derived from human induced pluripotent stem cells (hiPSCs) and their translation into a clinical-grade product for assisted reproduction.

The study demonstrates that hiPSC-derived OSCs significantly enhance the efficiency of in vitro maturation (IVM) of human oocytes, leading to improved embryo quality and pregnancy outcomes. Notably, the technology has already resulted in the birth of multiple healthy infants, suggesting a viable translational pathway for addressing long-standing limitations of IVM in clinical practice.
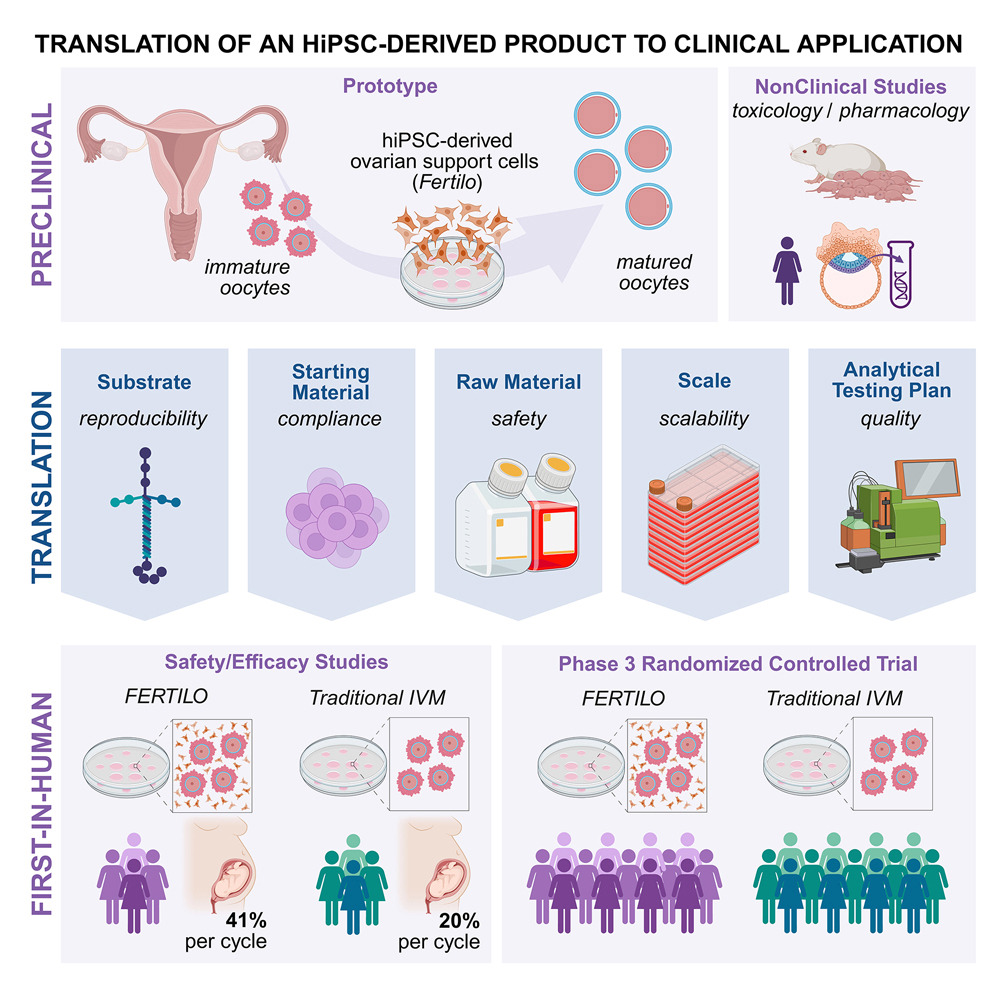
Global Infertility: An Unmet Medical Challenge
Infertility has become a major global public health concern. According to data from the World Health Organization (WHO), approximately 15–17% of individuals of reproductive age worldwide experience infertility. Conditions such as endometriosis, polycystic ovary syndrome (PCOS), and fertility impairment following cancer therapies impose not only substantial physical and psychological burdens on patients, but also significant socioeconomic costs.
Since the birth of the first IVF-conceived child in 1978, in vitro fertilization (IVF) has enabled millions of families to achieve pregnancy. However, conventional IVF protocols remain physically demanding. Patients typically undergo 10–14 days of high-dose gonadotropin stimulation to induce the maturation of multiple follicles, a process frequently associated with abdominal discomfort, nausea, vomiting, and, in severe cases, ovarian hyperstimulation syndrome (OHSS). While women under 35 may achieve first-cycle oocyte retrieval success rates of approximately 50%, success rates decline sharply with age, falling below 10% in women over 40—often necessitating repeated, costly treatment cycles.
IVM: A Gentler Alternative with Persistent Limitations
In vitro maturation (IVM) has long been considered a gentler alternative to conventional IVF. By allowing immature oocytes to complete maturation ex vivo, IVM minimizes or eliminates the need for high-dose hormonal stimulation and is particularly attractive for patients at elevated risk of OHSS, including those with PCOS.
Despite these advantages, the clinical adoption of IVM has been limited by consistently lower embryo quality and pregnancy success rates. A central limitation lies in the fundamentally static nature of conventional IVM culture systems, which fail to recapitulate the complex and dynamic signaling interactions between oocytes and ovarian support cells within the follicular microenvironment. The absence of granulosa cell–derived paracrine support is widely recognized as a key factor restricting IVM efficacy.
Against this backdrop, the researchers addressed a critical foundational question: Do hiPSC-derived cells truly acquire the molecular identity of ovarian support cells? Using transcription factor–guided differentiation with NR5A1, RUNX2, and GATA4, hiPSCs were reproducibly directed toward a granulosa-like phenotype. These cells robustly expressed canonical ovarian support cell markers, including FOXL2 and CD82, while pluripotency-associated markers such as OCT4 were effectively silenced. This molecular identity validation provided the necessary basis for subsequent functional studies.

TF-mediated hiPSC differentiation to generate OSCs that improve the rate of MII oocyte maturation
A Central Breakthrough: hiPSC-Derived OSCs Reconstruct the Ovarian Niche
To assess robustness and translational feasibility, the investigators evaluated the consistency and stability of OSCs across production batches. Single-cell transcriptomic analyses revealed a highly uniform population of mature OSCs, with minimal inter-batch variability—an essential requirement for clinical-grade manufacturing.
Functional gene expression profiling further demonstrated enrichment of signaling molecules and pathways intimately involved in oocyte maturation, including TGF-β, EGF, FGF2, IGF-related factors, as well as components of the NOTCH, BMP, and KITLG signaling pathways. These findings indicate that OSCs are not passive feeder cells, but instead actively engage in molecular crosstalk that supports oocyte development, while meeting the stringent reproducibility standards required for clinical translation.
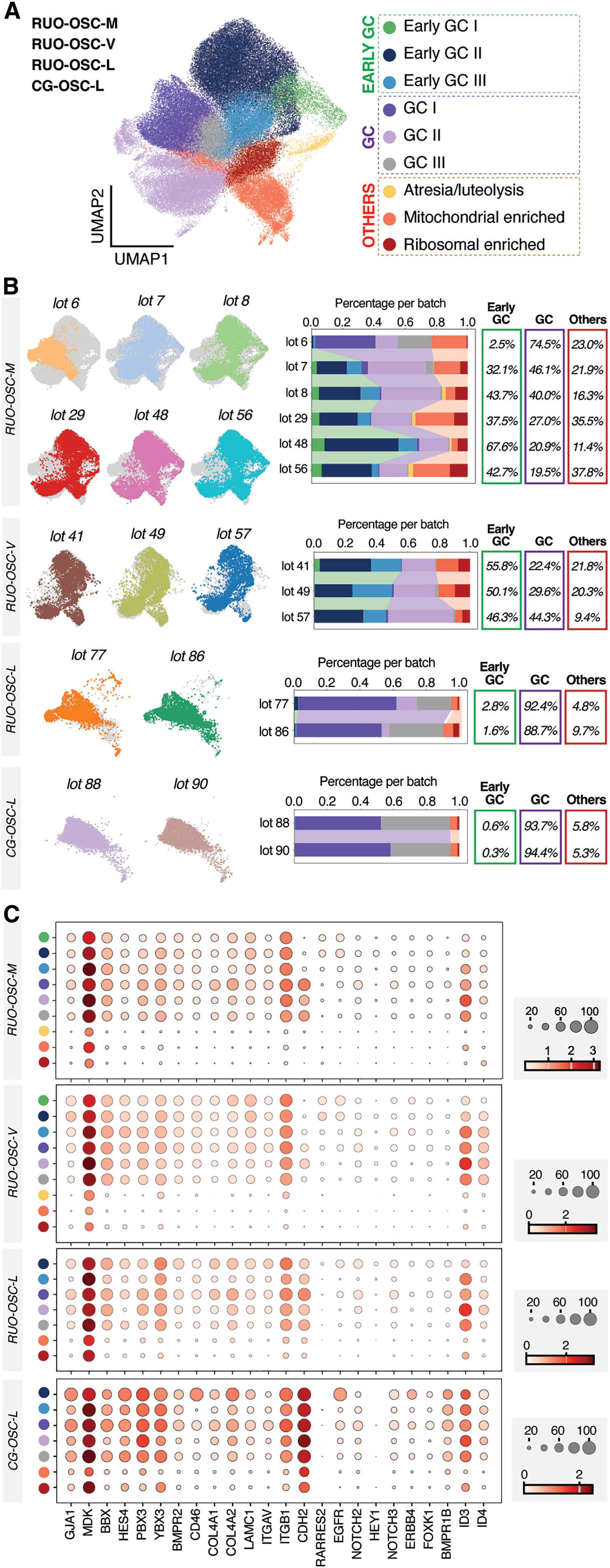
TF-mediated hiPSC differentiation by using CG materials leads to OSCs with consistent cellular outcomes
From Mechanism to Outcome: Clinical Evidence of Efficacy
The most compelling evidence supporting OSC-IVM comes from clinical outcome data. The study comprised two phases: an initial safety assessment in 20 patients, followed by a randomized controlled trial involving 20 patients assigned to either the Fertilo (OSC-IVM) group or a conventional IVM control group.
Use of the Fertilo platform increased oocyte maturation rates from 52% to 70%, doubled high-quality blastocyst formation rates from 7% to 14%, and increased the proportion of euploid blastocysts from 2% to 10%. These laboratory improvements translated into clinically meaningful outcomes, with a 41% clinical pregnancy rate in the Fertilo group compared to 20% in the conventional IVM cohort. At the time of reporting, eight healthy infants had been born following Fertilo treatment, versus two in the control group.
Collectively, these results establish a coherent evidence chain linking cellular identity, molecular function, embryo developmental competence, and clinical pregnancy outcomes, moving OSC-IVM beyond proof-of-concept toward validated clinical applicability.
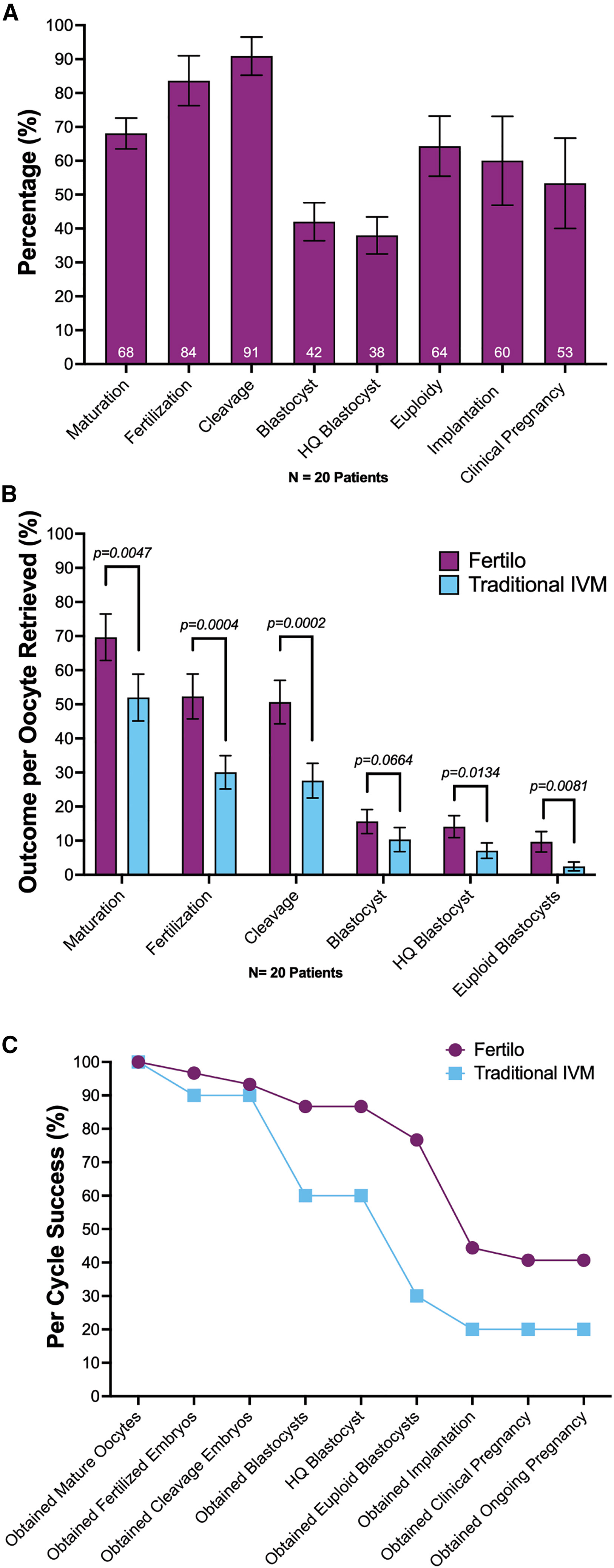
Clinical application of CG-OSCs significantly improves IVM outcomes
Scientific Significance: A Platform-Level Advancement
Beyond improving IVM success rates, this work introduces a scalable and standardized strategy for reconstructing the ovarian microenvironment ex vivo. It represents the first hiPSC-derived cell product applied to IVF and the first hiPSC-based therapy to advance to Phase III clinical trials in the United States. As such, it marks a pivotal step forward in the clinical translation of stem cell technologies within reproductive medicine.
Expanding Research Opportunities in Oocyte–Somatic Cell Interactions
As OSC-IVM technology advances, research into oocyte–support cell interactions, paracrine signaling networks, and key regulatory pathways governing follicular development is expected to accelerate. Addressing these questions requires access to well-characterized, high-quality research tools that support reproducible and mechanistically rigorous investigation.
From hiPSC differentiation to clinical-grade ovarian support cells, this study illustrates how fundamental stem cell research can reshape reproductive medicine. Advancing our understanding of oocyte maturation and the ovarian microenvironment begins with reliable experimental tools.
AntibodySystem: Supporting Reproductive and Stem Cell Research
AntibodySystem provides a comprehensive portfolio of research-grade tools to support studies focused on ovarian support cells and oocyte maturation mechanisms. For key molecular targets highlighted in this research—including FOXL2, CD82, TGF-β, EGFR, FGF2, IGF-related factors, and NOTCH pathway components—researchers can access high-quality recombinant proteins, monoclonal and polyclonal antibodies, and ELISA kits validated for applications such as Western blotting (WB), immunofluorescence (IF), immunohistochemistry (IHC), ELISA, and functional assays. These solutions are designed to support reproducible results and robust mechanistic insights.
|
Catalog |
Product Name |
|
EHB92502 |
Recombinant Human FSH Dimer Protein, C-His |
|
YHC71101 |
Recombinant Human CGB3 Protein, N-His |
|
RHC38202 |
Anti-FGF2/FGFb Antibody (R3B46) |
|
RHD74901 |
Anti-Human CD82 Antibody (SAA1615) |
|
RHF90001 |
Anti-POU5F1/OCT3/OCT4 Antibody (R3Q45) |
|
PHF24901 |
Anti-Human FOXL2 Polyclonal Antibody |
|
VMB91601 |
InVivoMAb Anti-Human/Mouse TGF-β Antibody (1D11.16.8) |
