A recent study published in Nature, entitled “Sustained HIV-1 remission after heterozygous CCR5Δ32 stem cell transplantation,” reports a case of long-term HIV-1 remission in a patient who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) from a donor carrying a heterozygous CCR5Δ32 mutation.

This study provides new clinical evidence supporting the concept of functional HIV cure and further deepens our understanding of the role of CCR5 as a key host factor in sustained viral control.
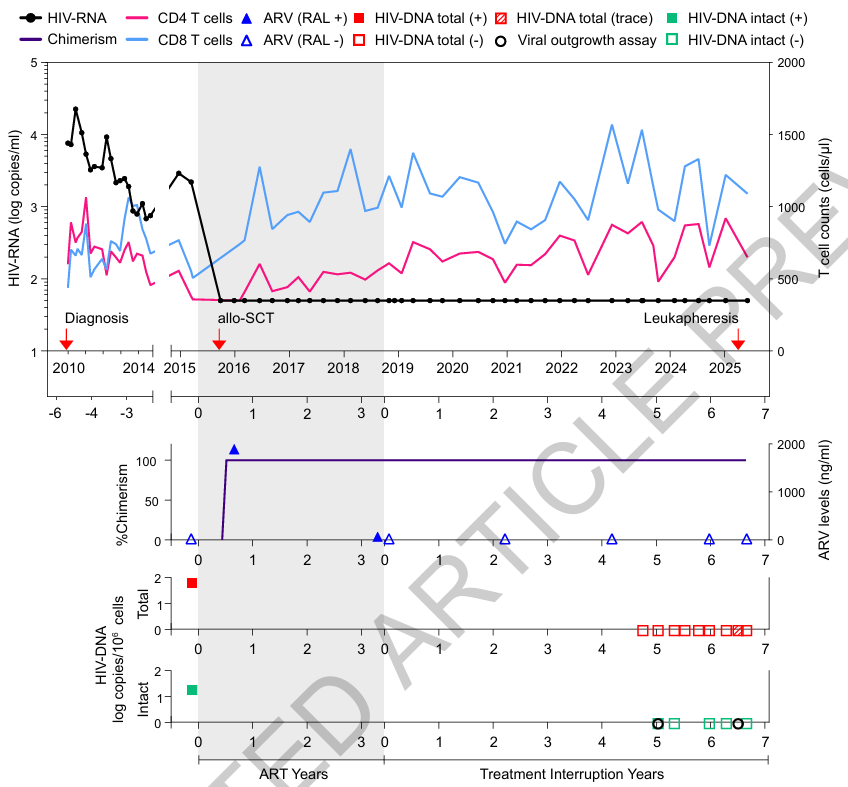
Figure 1. Clinical timeline of allogeneic stem cell transplantation, ART interruption, and longitudinal HIV monitoring.
Background: CCR5 in HIV-1 Infection
HIV-1 entry into CD4⁺ T cells is primarily mediated by the co-receptors CCR5 or CXCR4, with CCR5 serving as the dominant entry pathway for R5-tropic HIV-1 strains. Previously reported cases of long-term HIV remission following stem cell transplantation have predominantly involved donors with homozygous CCR5Δ32 mutations, resulting in the absence of CCR5 expression and thereby restricting viral entry into newly generated target cells.
However, homozygous CCR5Δ32 mutations are extremely rare in the general population, significantly limiting the broader applicability of such approaches. Consequently, whether complete CCR5 deficiency is strictly required to achieve sustained HIV control has remained an important and unresolved question in the field.
Key Findings: Virological and Immunological Evidence After Heterozygous CCR5Δ32 Transplantation
In the present study, the patient underwent allo-HSCT for a hematological malignancy using a donor carrying a single CCR5Δ32 allele (heterozygous). Following transplantation, the patient’s hematopoietic system was progressively reconstituted with donor-derived cells, and peripheral blood analyses demonstrated a highly stable donor chimerism. Newly generated CD4⁺ T cells were predominantly of donor origin.
After discontinuation of antiretroviral therapy (ART) under strict clinical monitoring, the patient was subjected to comprehensive longitudinal virological assessments. These included measurements of plasma HIV-1 RNA, quantification of HIV DNA in peripheral CD4⁺ T cells, and assays for replication-competent virus. Throughout the follow-up period, no viral rebound was detected, and no replication-competent HIV was recovered.
Importantly, although CCR5 expression was not completely absent, both the expression level of CCR5 and the proportion of CCR5⁺ target cells were markedly reduced in newly generated CD4⁺ T cells. These findings suggest that even in the presence of residual CCR5 expression, immune reconstitution combined with reduced target cell availability may be sufficient to impose durable constraints on HIV-1 replication.
Implications and Cautious Interpretation
As emphasized by the authors, this case does not indicate that heterozygous CCR5Δ32 status alone is sufficient to reproducibly achieve HIV cure. Rather, the observed remission is likely the result of multiple converging mechanisms, including donor-derived immune system reconstitution, altered CCR5 expression dynamics, and transplant-associated immune effects on the viral reservoir.
Accordingly, the clinical significance of this study lies primarily in its role as a proof of concept, offering important insights for the development of CCR5-targeted gene editing, cell-based therapies, and immune intervention strategies, rather than representing a broadly applicable therapeutic approach.
Broader Context in HIV Cure Research
In parallel, Nature published two additional HIV studies highlighting that synergistic interactions between broadly neutralizing antibodies (bNAbs), vaccination strategies, and CD8⁺ T cell–mediated immune activation can sustain viral control after treatment interruption. Collectively, these findings reinforce the emerging consensus that durable HIV remission is likely to depend on multidimensional immune mechanisms, rather than single-target interventions.
AntibodySystem provides HIV1-related products, delivering more tools and solutions for research.
|
Catalog |
Product Name |
|
EVV03101 |
Recombinant HIV1 GP140 Protein, C-His |
|
EVV07801 |
Recombinant HIV1 gp120/SU Protein, C-His |
|
YVV36301 |
Recombinant HIV1 Protein Tat Protein, N-GST & C-His |
|
YVV19301 |
Recombinant HIV-1 p24/Capsid protein p24 Protein, N-His-SUMO |
|
YHF39901 |
Recombinant Human CD184/CXCR4 Protein, N-His |
|
RVV23901 |
Anti-HIV-1 nef/F-protein protein Nanobody (SAA1423) |
|
RVV23801 |
Anti-HIV-1 rev/ART/TRS protein Nanobody (SAA1422) |
|
RVV19304 |
Anti-HIV-1 Matrix protein p17/MA protein Antibody (M33) |
|
RVV19301 |
Anti-HIV1 Gag polyprotein Nanobody (SAA0889) |
|
RVV03101 |
Anti-HIV1 Surface protein gp120 Nanobody (SAA0912) |
|
RVV03102 |
Anti-HIV1 Surface protein gp120 Nanobody (SAA0881) |
|
DVV03106 |
Research Grade Anti-HIV1 gp120/Glycoprotein 120 (VRC01LS) |
|
DVV07801 |
Research Grade Anti-HIV gp120SU (VRC01) |
|
VVV03108 |
InVivoMAb Anti-HIV1 env/Env polyprotein Antibody (CH59) |
|
VVV03105 |
InVivoMAb Anti-HIV1 env/Env polyprotein Broadly Neutralizing Antibody (Iv0114) |
|
FHE85412 |
Anti-Human CD195/CCR5 Antibody (PRO-140), PE |
|
FHE85422 |
Anti-Human Sulfated CD195/CCR5 Antibody (RoAb13), PE |
|
FHE85432 |
Anti-Human CCR5 Antibody (ST6), PE |
|
FHE85414 |
Anti-Human CD195/CCR5 Antibody (PRO-140), PerCP |
|
FHF39910 |
Anti-Human CD184/CXCR4 Antibody (SAA0067) |
|
FHE85410 |
Anti-Human CD195/CCR5 Antibody (PRO-140) |
|
DHF39902 |
Research Grade Anti-Human CD184/CXCR4 Antibody (MEDI3185) |
|
FHF39920 |
Anti-Human CD184/CXCR4 Antibody (SAA0068) |
|
RHF39902 |
Anti-CD184/CXCR4 Antibody (R1B46) |
