In October 2025, AntibodySystem products continues to gain recognition within the global research community, with our products cited in recent publications across prestigious journals including Advanced Science and Cell Reports. The research encompasses several cutting-edge areas, showcasing the critical role our reagents play in advancing both basic and translational life science research—from novel therapeutic strategies for Graves' ophthalmopathy and uncovering mechanisms in temporal lobe epilepsy, to elucidating mpox virus replication, developing antibodies against emerging henipaviruses, dissecting the tumor microenvironment in colorectal cancer, and characterizing the functional constraints of the Nipah virus fusion protein.
This broad application underscores the critical value of our products in supporting both fundamental life science research and translational medical exploration. Moving forward, AntibodySystem remains committed to collaborating with research teams worldwide, facilitating the entire research pipeline from molecular mechanism elucidation to the identification of novel therapeutic targets, collectively pushing the boundaries of science. Now, let's explore some of the newly added citation literature from October with Dr. Connie!

|
Title |
TSHR-Targeting Nucleic Acid Aptamer Treats Graves' Ophthalmopathy via Novel Allosteric Inhibition |
|
Journal Information |
Adv Sci (Weinh). 2025 Oct 7:e05586. |
|
Cited products |
|
|
Catalog |
Product Name |
|
DHC29903 |
Research Grade Teprotumumab |
Key finding:
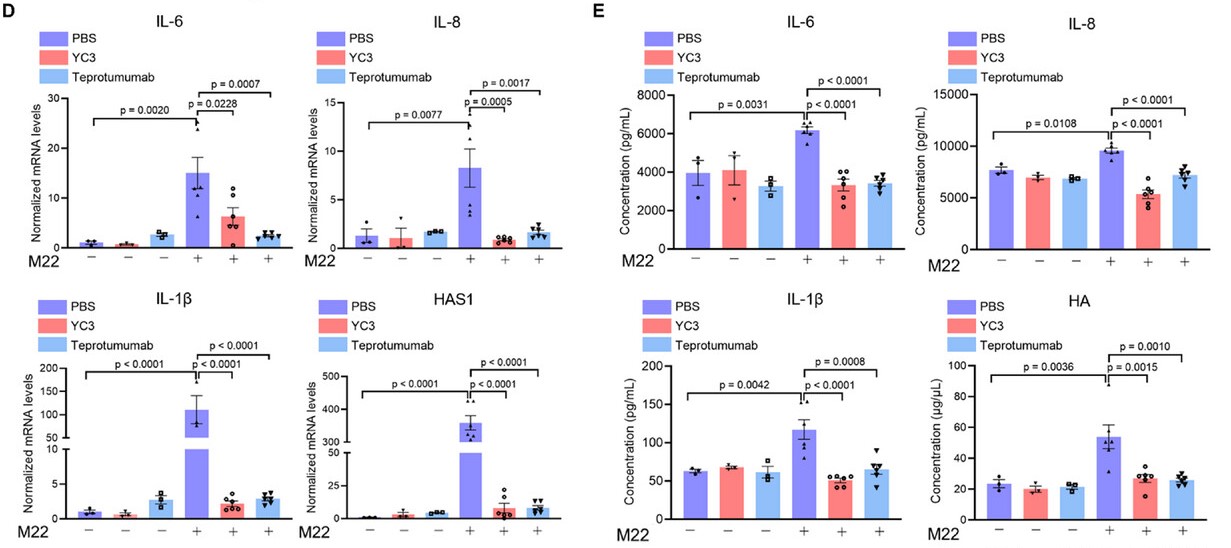
Graves’ ophthalmopathy (GO) is an autoimmune disorder driven by thyrotropin receptor (TSHR) autoantibodies, leading to orbital inflammation. Current treatments are palliative, highlighting a need for targeted therapies. Researchers developed YC3, a TSHR-targeting nucleic acid aptamer. It potently reversed disease-associated signaling in human orbital fibroblasts and alleviated symptoms in a GO mouse model. Mechanistically, YC3 works by binding a novel allosteric site on TSHR, inhibiting its activation. This study presents YC3 as a promising therapeutic and unveils a new druggable site, demonstrating aptamers' value in both drug discovery and mechanistic research.

This study cited AntibodySystem's Research Grade Teprotumumab (Catalog #DHC29903) as a positive control, comparing its effects with those of YC3. The antibody showed significant inhibition of inflammatory responses and hyaluronic acid synthesis in Graves' orbital fibroblasts, further validating its potential as an effective biologic for treating Graves' ophthalmopathy.

|
Title |
Elevated Apolipoprotein E Expression in Hippocampal Microglia Drives Temporal Lobe Epilepsy Progression |
|
Journal Information |
Adv Sci (Weinh). 2025 Oct 14:e05778. |
|
Cited products |
|
|
Catalog |
Product Name |
|
RMB98601 |
Anti-Mouse APOE Antibody (HJ6.3) |
Key finding:
Temporal lobe epilepsy (TLE) is often associated with hippocampal sclerosis (HS), where microglial cells play a key role. This study identifies apolipoprotein E (APOE) as a critical driver of TLE progression. APOE expression is significantly elevated in TLE, primarily within a specific subset of hippocampal microglia. Functional experiments demonstrate that these APOE-expressing microglia promote neuroinflammation, neuronal hyperexcitability, and cell death. Genetically knocking out APOE in a mouse model alleviated gliosis, seizures, and neuronal loss. The findings establish APOE and its downstream signaling pathways as promising therapeutic targets for TLE.

This study cited AntibodySystem's Anti-Mouse APOE Antibody (HJ6.3) (Catalog #RMB98601) used as an APOE inhibitor in in vitro cell experiments. Results demonstrated that neutralizing APOE protein function significantly suppressed neuroinflammatory responses in microglia and the activation of related signaling pathways.


|
Title |
Blocking Lysine Crotonylation and Aerobic Glycolysis as Targeting Strategy Against mpox Virus Replication |
|
Journal Information |
Adv Sci (Weinh). 2025 Oct 27:e09148. |
|
Cited products |
|
|
Catalog |
Product Name |
|
PVV14801 |
Anti-Monkeypox virus/MPXV F3L Polyclonal Antibody |
|
PVV13201 |
Anti-Monkeypox virus/MPXV E8L Polyclonal Antibody |
Key finding:
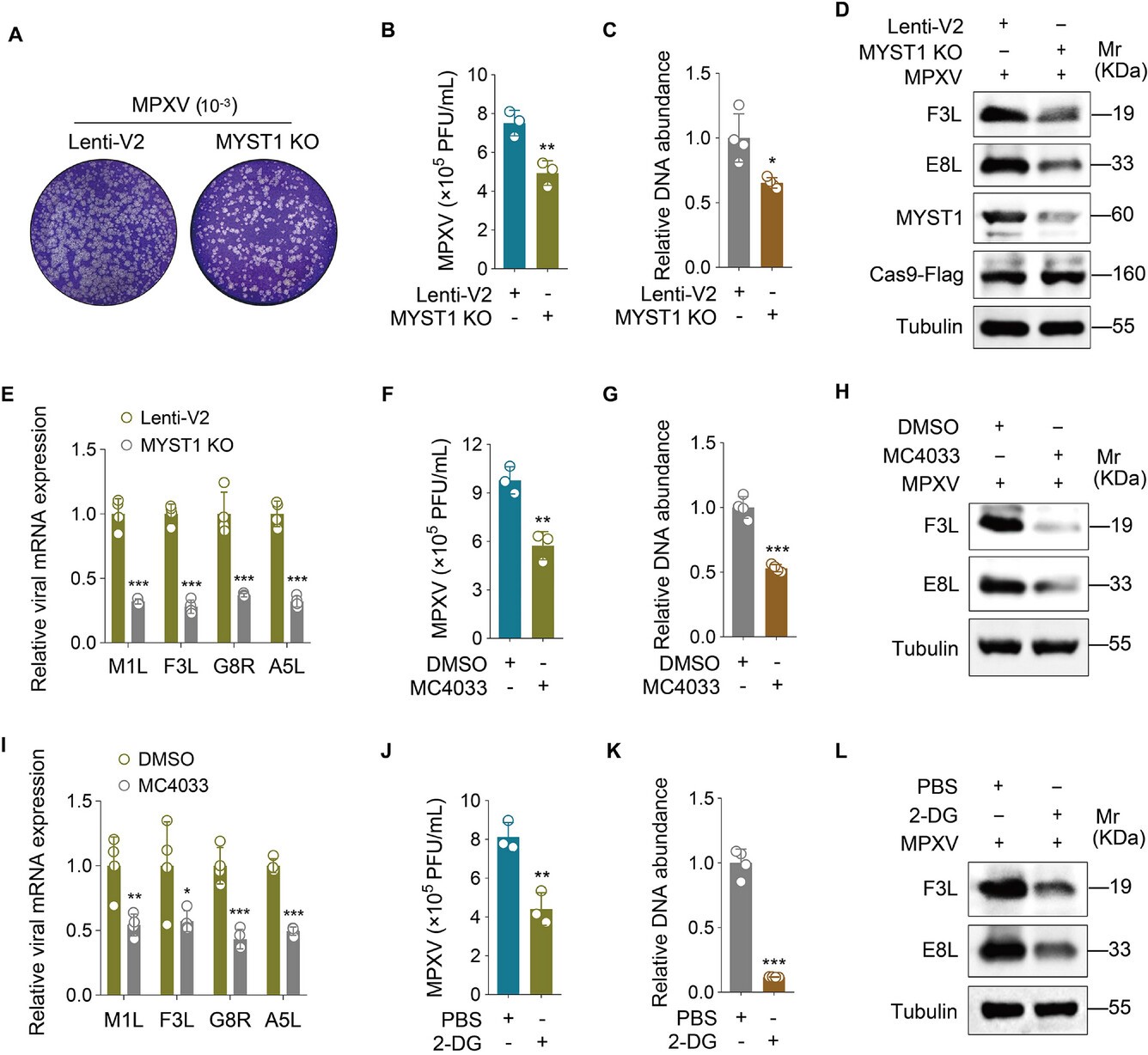
The mpox virus (MPXV) reprograms host cell metabolism to facilitate its replication. This study reveals that MPXV hijacks aerobic glycolysis through a novel mechanism: the viral I3 protein undergoes lysine crotonylation, a modification catalyzed by the host enzyme MYST1. This crotonylated I3 then stabilizes the protein WDR26, which in turn enhances aerobic glycolysis and promotes viral replication. Critically, inhibiting this pathway—either by blocking MYST1 with the drug MC4033 or by using glycolytic inhibitors like 2-DG—effectively suppresses MPXV replication. These findings identify viral protein crotonylation and host glycolysis as promising therapeutic targets against mpox.

This study cited AntibodySystem's Anti-Monkeypox virus/MPXV F3L Polyclonal Antibody (Catalog #PVV14801) and Anti-Monkeypox virus/MPXV E8L Polyclonal Antibody (Catalog #PVV13201) for Western Blot experiments, confirming that targeting MYST1 or aerobic glycolysis effectively inhibits MPXV viral protein expression and viral replication.

|
Title |
Structure and function of a pair of non-competing monoclonal antibodies against Langya henipavirus attachment glycoprotein |
|
Journal Information |
Cell Rep. 2025 Oct 9;44(10):116407. |
|
Cited products |
|
|
Catalog |
Product Name |
|
PVV18301 |
Anti-Langya virus/LayV G/Glycoprotein Polyclonal Antibody |
Key finding:
Langya henipavirus (LayV) is an emerging zoonotic virus. Its attachment glycoprotein (G), essential for cell entry, shows significant antigenic differences from related Hendra and Nipah viruses, suggesting existing therapies may be ineffective. To address this, researchers immunized mice with the LayV-G ectodomain and isolated a panel of monoclonal antibodies (mAbs). They identified two potent mAbs with strong antiviral function. Using cryo-electron microscopy, the team determined the structure of these mAbs bound to distinct epitopes on the LayV-G head domain. This work reveals the antibody recognition mechanisms and defines two key functional sites on LayV-G, providing a foundation for developing effective therapeutics and vaccines against this emerging pathogen.

This study cited AntibodySystem's Anti-Langya virus/LayV G/Glycoprotein Polyclonal Antibody (Catalog #PVV18301), primarily used as a critical positive control reagent in WB and ELISA experiments to validate the experimental system's reliability and aid in characterizing the newly isolated monoclonal antibodies.
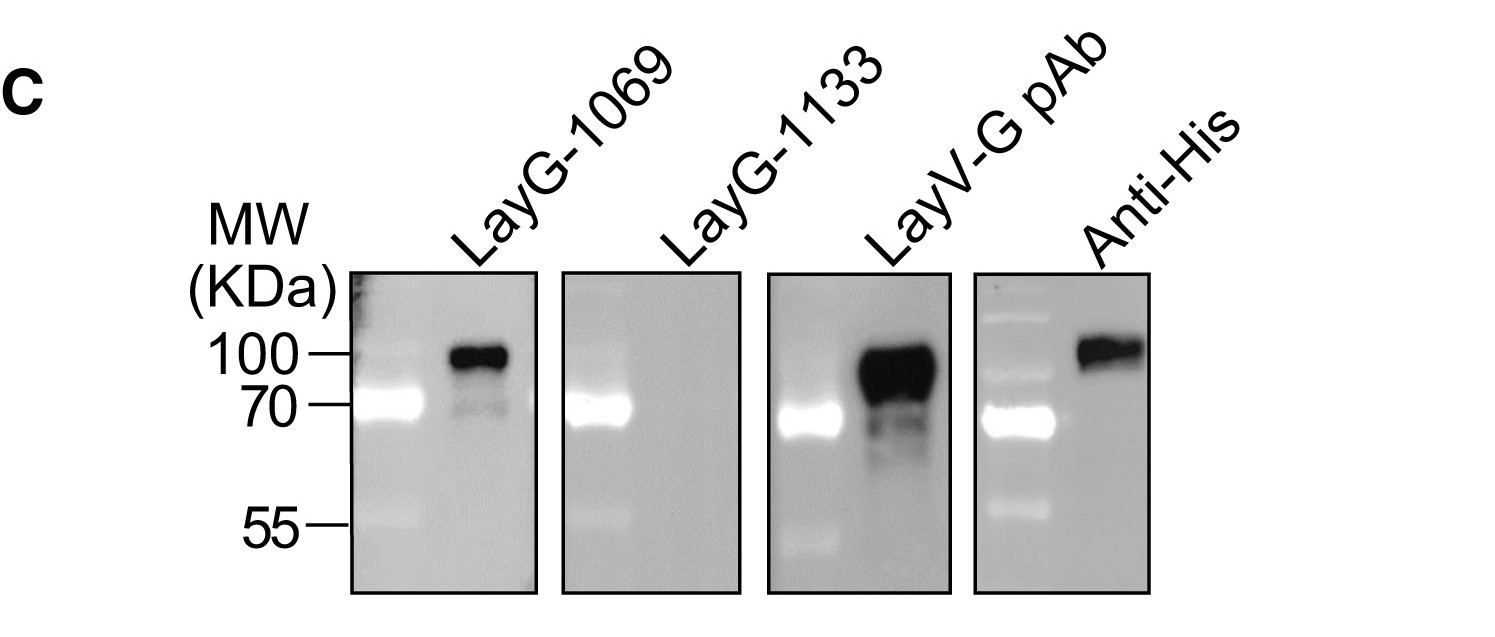

|
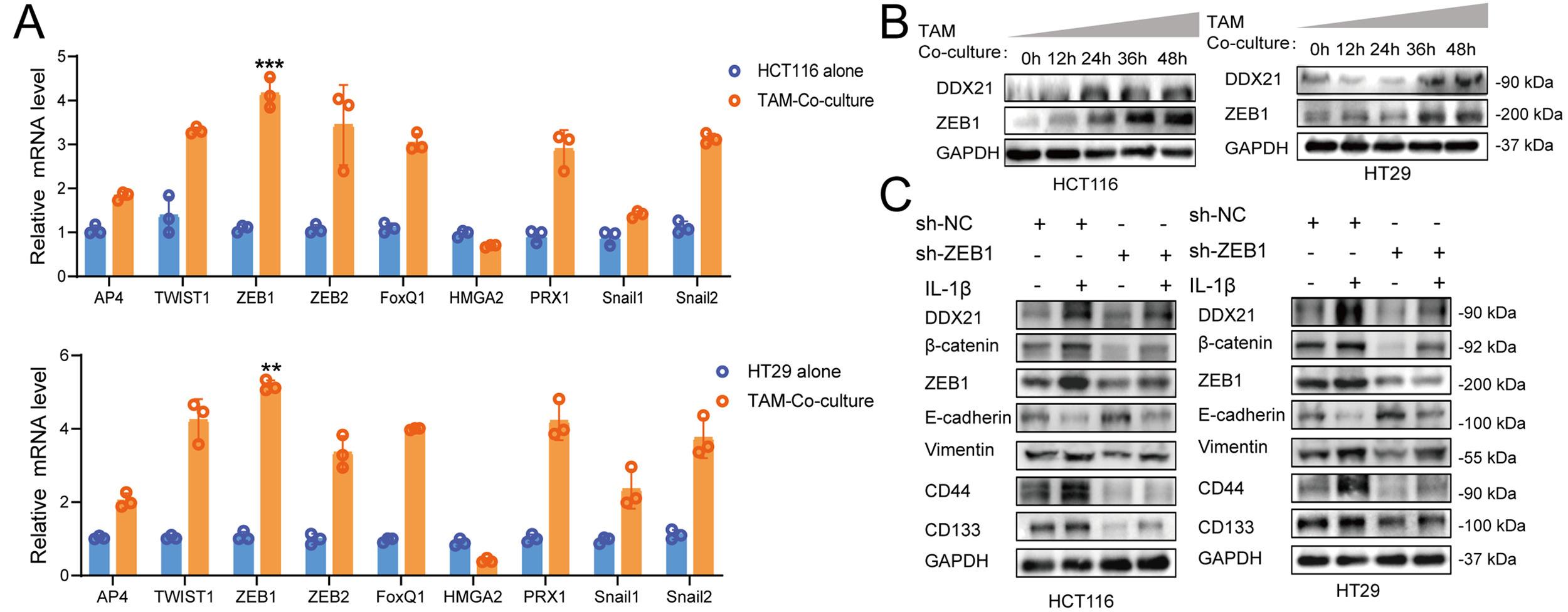
Title |
TAMs-derived IL-1β inducing DDX21 enhances CRC proliferation and metastasis via JAK2/STAT3 pathway |
|
Journal Information |
Cancer Treat Res Commun. 2025 Oct 20:45:101022. |
|
Cited products |
|
|
Catalog |
Product Name |
|
VHC79101 |
InVivoMAb Anti-Human IL8/CXCL8 (Iv0023) |
|
VHB94401 |
InVivoMAb Anti-Human TNFa/TNF-alpha (Iv0050) |
|
VHB95601 |
InVivoMAb Anti-Human IL1B/IL1F2 (Iv0019) |
|
VHF70301 |
InVivoMAb Anti-Human CCL8/MCP-2 (Iv0080) |
Key finding:
This study reveals a critical feedback loop between colorectal cancer (CRC) cells and tumor-associated macrophages (TAMs). We found that TAMs promote CRC proliferation, metastasis, and stemness by secreting IL-1β, which activates the JAK2/STAT3 pathway in cancer cells. Activated STAT3 binds to and stabilizes the DDX21 protein. DDX21, in turn, upregulates ZEB1 expression, leading to the secretion of the chemokine CCL8. CCL8 recruits more macrophages, thereby sustaining the loop. In vivo, IL-1β knockdown impaired TAM-induced tumorigenesis. Clinically, high levels of IL-1β and the TAM marker CD206 correlate with poor patient prognosis. These findings identify TAM-derived IL-1β as a promising immunotherapeutic target for CRC.

This study cited AntibodySystem's InVivoMAb Anti-Human IL8/CXCL8 (Iv0023) (Catalog #VHC7910), InVivoMAb Anti-Human TNFa/TNF-alpha (Iv0050)(Catalog #VHB94401), InVivoMAb Anti-Human IL1B/IL1F2 (Iv0019)(Catalog #VHB95601), and InVivoMAb Anti-Human CCL8/MCP-2 (Iv0080) (Catalog #VHF70301), primarily used in Neutralizing Antibody experiments to validate the roles of TAMs-secreted cytokines (e.g., IL-1β, CCL8) in regulating DDX21 expression, ZEB1-mediated EMT, stem cell properties, and macrophage recruitment. Results indicated the core role of the IL-1β/STAT3/DDX21/ZEB1/CCL8 axis in CRC malignant progression and immune microenvironment regulation, while TNF-α and IL-8 were not significantly involved in this specific pathway.

|
Title |
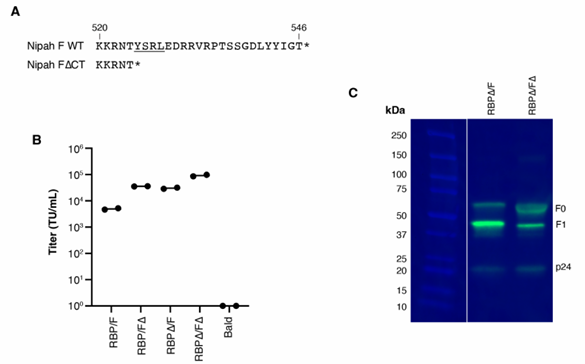
Functional and antigenic constraints on the Nipah virus fusion protein |
|
Journal Information |
bioRxiv. October 15, 2025 |
|
Cited products |
|
|
Catalog |
Product Name |
|
PVV08101 |
Anti-Nipah virus/HeV F/Fusion glycoprotein F0 Polyclonal Antibody |
Key finding:
Nipah virus employs two glycoproteins for infection: the receptor-binding protein (RBP) and the fusion protein (F). Using pseudoviruses, we systematically analyzed the functional effects of all single-amino-acid mutations in the F protein. Results show that F is significantly more functionally constrained than the RBP. We identified critical mutation-intolerant sites on the F trimer surface and core, essential for its function, and identified mutations that may stabilize the prefusion conformation for vaccine design. We also quantified how F mutations affect neutralization by six monoclonal antibodies, revealing varying impacts across antibodies. These mutational effects on neutralization even predicted cross-neutralization efficacy against the related Hendra virus. This work comprehensively defines the functional and antigenic constraints on this key zoonotic viral protein.

This study cited AntibodySystem's Anti-Nipah virus/HeV F/Fusion glycoprotein F0 Polyclonal Antibody (Catalog #PVV08101) in WB experiments to validate the expression and cleavage of cytoplasmically tail-truncated Nipah virus F protein in the pseudovirus system. Experiments confirmed that the truncated F protein was effectively cleaved to generate the F1 subunit and successfully incorporated into viral particles, supporting the reliability of the subsequent deep mutational scanning system.